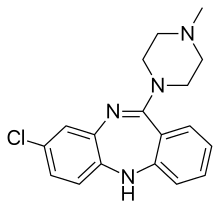
Clozapine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Clozaril, FazaClo, Versacloz |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a691001 |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | N05AH02 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60 to 70% |
| Metabolism | Liver, by several CYP isozymes |
| Biological half-life | 6 to 26 hours (mean value 14.2 hours in steady state conditions) |
| Excretion | 80% in metabolized state: 30% biliary and 50% kidney |
| Identifiers | |
| |
| CAS Number |
5786-21-0 |
| PubChem (CID) | 2818 |
| IUPHAR/BPS | 38 |
| DrugBank |
DB00363 |
| ChemSpider |
10442628 |
| UNII |
J60AR2IKIC |
| KEGG |
D00283 |
| ChEBI |
CHEBI:3766 |
| ChEMBL |
CHEMBL42 |
| ECHA InfoCard | 100.024.831 |
| Chemical and physical data | |
| Formula | C18H19ClN4 |
| Molar mass | 326.823 g/mol |
| 3D model (Jmol) | Interactive image |
| Melting point | 183 °C (361 °F) |
| Solubility in water | 0.1889[2] mg/mL (20 °C) |
| |
| |
| (verify) | |
Clozapine, sold under the brand name Clozaril among others, is an atypical antipsychotic medication.[1] It is mainly used for schizophrenia that does not improve following the use of other antipsychotic medications. In those with schizophrenia and schizoaffective disorder it may decrease the rate of suicidal behavior.[1] It is possibly more effective than typical antipsychotics and in those who are treatment resistant.[3][4] It is taken by mouth.[1]
Clozapine is associated with a relatively high risk of low white blood cells which may result in death. To decrease this risk it is recommended that the blood be regularly monitored.[1] Other serious risks include seizures, inflammation of the heart, high blood sugar levels, and in older people with psychosis as a result of dementia an increased risk of death.[1][5] Common side effects include drowsiness, dry mouth, low blood pressure, trouble seeing, and dizziness.[1] The potentially permanent movement disorder tardive dyskinesia occurs in about 5% of people.[5] Its mechanism of action is not entirely clear.[1]
Clozapine was first made in 1958 and sold commercially in 1972.[6] It was the first atypical antipsychotic.[7] It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[8] It is available as a generic medication.[1] The wholesale cost in the developing world is between 0.05 and 2.10 USD per day as of 2014.[9]
Medical uses
Clozapine is an atypical antipsychotic drug primarily used in people who are unresponsive to or intolerant of other antipsychotics.[10] This means that they have failed to respond satisfactorily to at least two different antipsychotics.[11] It has been shown to be more effective in reducing symptoms of schizophrenia than typical antipsychotics, with more pronounced effects in those who have responded poorly to other medication. The relapse rate is lower and patient acceptability is better.[10] There is some evidence clozapine may reduce propensity for substance abuse in schizophrenic patients.[12]
It may be better than other antipsychotics in people with both schizophrenia and Parkinson's.[13]
Clozapine is not recommended for the treatment of behavior problems in older adults with dementia.
Side effects
Clozapine may cause side effects. Some are serious and potentially fatal. Common side effects include constipation, bed-wetting, night-time drooling, muscle stiffness, sedation, tremors, orthostatic hypotension, hyperglycemia, and weight gain. The risk of developing extrapyramidal symptoms such as tardive dyskinesia is below that of typical antipsychotics; this may be due to clozapine's anticholinergic effects. Extrapyramidal symptoms may subside somewhat after a person switches from another antipsychotic to clozapine.[14]
Clozapine also carries five black box warnings, including warnings for agranulocytosis, CNS depression, leukopenia, neutropenia, seizure disorder, bone marrow suppression, dementia, hypotension, myocarditis, orthostatic hypotension (with or without syncope) and seizures.[15] Lowering of the seizure threshold may be dose related and slow initial titration of dose may decrease the risk for precipitating seizures. Slow titration of dosing may also decrease the risk for orthostatic hypotension and other adverse cardiovascular side effects.[16]
Many male patients have experienced cessation of ejaculation during orgasm as a side effect of clozapine, though this is not documented in official drug guides.[17]
However, many side-effects can be managed and do not necessarily warrant discontinuation.[18]
Agranulocytosis
Clozapine carries a black box warning for drug-induced agranulocytosis. Without monitoring, agranulocytosis occurs in about 1% of patients who take clozapine during the first few months of treatment;[19] the risk of developing it is highest about three months into treatment, and decreases substantially thereafter, to less than 0.01% after one year.[20]
Clozapine-induced agranulocytosis can be transient.[21]
Cardiac toxicity
Myocarditis is a sometimes fatal side effect of clozapine, which usually develops within the first month of commencement.[22] First manifestations of illness are fever which may be accompanied by symptoms associated with upper respiratory tract, gastrointestinal or urinary tract infection. Typically C-reactive protein (CRP) increases with the onset of fever and rises in the cardiac enzyme, troponin, occur up to 5 days later. Monitoring guidelines advise checking CRP and troponin at baseline and weekly for the first 4 weeks after clozapine initiation and observing the patient for signs and symptoms of illness.[23] Signs of cardiac failure are less common and may develop with the rise in troponin. A recent case-control study found that the risk of clozapine-induced myocarditis is increased with increasing rate of clozapine dose titration, increasing age and concomitant sodium valproate.[24]
Gastrointestinal hypomotility
Another underrecognized and potentially life-threatening side effect spectrum is gastrointestinal hypomotility, which may manifest as severe constipation, fecal impaction, paralytic ileus, bowel obstruction, acute megacolon, ischemia or necrosis. Colonic hypomotility has been shown to occur in up to 80% of people prescribed clozapine when gastrointestinal function is measured objectively using radiopaque markers.[25] Monitoring of bowel function is recommended, as untreated cases are occasionally fatal.[26][27]
Hypersalivation
While clozapine is a muscarinic antagonist at the M1, M2, M3, and M5 receptors, clozapine is a full agonist at the M4 subset. Because M4 is highly expressed in the salivary gland, its M4 agonist activity is thought to be responsible for the hypersalivation.[28]
Central nervous system
CNS side effects include drowsiness, vertigo, headache, tremor, syncope, sleep disturbances, nightmares, restlessness, akinesia, agitation, seizures, rigidity, akathisia, confusion, fatigue, insomnia, hyperkinesia, weakness, lethargy, ataxia, slurred speech, depression, myoclonic jerks, and anxiety. Rarely seen are delusions, hallucinations, delirium, amnesia, libido increase or decrease, paranoia and irritability, abnormal EEG, worsening of psychosis, paresthesia, status epilepticus, and obsessive compulsive symptoms. Similar to other antipsychotics clozapine rarely has been known to cause neuroleptic malignant syndrome.[29]
Urinary incontinence
Clozapine is linked to urinary incontinence[30] though its appearance may be under-recognized.[31]
Withdrawal effects
Abrupt withdrawal may lead to cholinergic rebound effects, severe movement disorders as well as severe psychotic decompensation. It has been recommended that patients, families, and caregivers are aware of the symptoms and risks of abrupt withdrawal of clozapine. When discontinuing clozapine, gradual dose reduction is recommended to reduce the intensity of withdrawal effects.[32][33]
Weight gain and diabetes
In addition to hyperglycemia, significant weight gain is frequently experienced by patients treated with clozapine.[34] Impaired glucose metabolism and obesity have been shown to be constituents of the metabolic syndrome and may increase the risk of cardiovascular disease. The data suggest that clozapine may be more likely to cause adverse metabolic effects than some of the other atypical antipsychotics.[35] A study has established that olanzapine and clozapine disturb the metabolism by making the body take preferentially its energy from fat (instead of privileging carbohydrates). Levels of carbohydrates remaining high, the body develops insulin resistance (causing diabetes).[36]
Interactions
Fluvoxamine inhibits the metabolism of clozapine leading to significantly increased blood levels of clozapine.[37]
When carbamazepine is concurrently used with clozapine, it has been shown to decrease plasma levels of clozapine significantly thereby decreasing the beneficial effects of clozapine.[38][39] Patients should be monitored for “decreased therapeutic effects of clozapine if carbamazepine” is started or increased. If carbamazepine is discontinued or the dose of carbamazepine is decreased, therapeutic effects of clozapine should be monitored. The study recommends carbamazepine to not be used concurrently with clozapine due to increased risk of agranulocytosis.[40]
Published case reports have stated that the use of benzodiazepines and clozapine concomitantly can result in severe adverse reaction such as respiratory arrest, cardiac arrest and sudden death.[41]
Ciprofloxacin is an inhibitor of CYP1A2 and clozapine is a major CYP1A2 substrate. Randomized study reported elevation in clozapine concentration in schizophrenia subjects concurrently taking ciprofloxacin.[42] Thus, the prescribing information for clozapine recommends “reducing the dose of clozapine by one-third of original dose” when ciprofloxacin and other CYP1A2 inhibitors are added to therapy, but once ciprofloxacin is removed from therapy, it is recommended to return clozapine to original dose.[43]
Chemistry
Clozapine is a dibenzodiazepine that is structurally related to loxapine. It is slightly soluble in water, soluble in acetone, and highly soluble in chloroform. Its solubility in water is 188.9 mg/L (25 °C).[2] Its manufacturer, Novartis, claims a solubility of <0.01% in water (<100 mg/L).[44]
Mechanism of action
Clozapine is classified as an atypical antipsychotic drug because it binds to serotonin as well as dopamine receptors.[45]
Clozapine is a partial agonist[46] at the 5-HT1A subunit of the serotonin receptor, putatively improving depression, anxiety, and the negative cognitive symptoms associated with schizophrenia.[47]
A direct interaction of clozapine with the GABAB receptor has also been shown.[48] GABAB receptor-deficient mice exhibit increased extracellular dopamine levels and altered locomotor behaviour equivalent to that in schizophrenia animal models.[49] GABAB receptor agonists and positive allosteric modulators reduce the locomotor changes in these models.[50]
Clozapine induces the release of glutamate and D-serine, an agonist at the glycine site of the NMDA receptor, from astrocytes,[51] and reduces the expression of astrocytic glutamate transporters. These are direct effects that are also present in astrocyte cell cultures not containing neurons. Clozapine prevents impaired NMDA receptor expression caused by NMDA receptor antagonists.[52]
Binding Affinity (Ki [nM]) towards cloned human receptors unless otherwise specified[53]
| Receptor/Transporter Protein | Clozapine | Norclozapine |
|---|---|---|
| SERT | 1624 | 316.6 |
| NET | 3168 | 493.9 |
| DAT | >10,000 | >10,000 |
| 5-HT1A | 123.7 | 13.9 |
| 5-HT1B | 519 | 406.8 |
| 5-HT1D | 1356 | 476.2 |
| 5-HT2A | 5.35 | 10.9 |
| 5-HT2B | 8.37 | 2.8 |
| 5-HT2C | 9.44 | 11.9 |
| 5-HT3 | 241 | 272.2 |
| 5-HT5A | 3857 | 350.6 |
| 5-HT6 | 13.49 | 11.6 |
| 5-HT7 | 17.95 | 60.1 |
| D1 | 266.25 | 14.3 |
| D2 | 157 | 101.4 |
| D3 | 269.08 | 193.5 |
| D4 | 26.36 | 63.94 |
| D5 | 255.33 | 283.6 |
| α1A | 1.62 | 104.8 |
| α1B | 7 | 85.2 |
| α2A | 37 | 137.6 |
| α2B | 26.5 | 95.1 |
| α2C | 6 | 117.7 |
| β1 | 5000 | 6239 |
| β2 | 1650 | 4725 |
| M1 | 6.17 | 67.6 |
| M2 | 36.67 | 414.5 |
| M3 | 19.25 | 95.7 |
| M4 | 15.33 | 169.9 |
| M5 | 15.5 | 35.4 |
| H1 | 1.13 | 3.4 |
| H2 | 153 | 345.1 |
| H3 | >10,000 | >10,000 |
| H4 | 665 | 1028 |
| δ-opioid | 1000 (Mouse receptor) | 127.9 |
| μ-opioid | 1000 (Rat receptor) | >10,000 |
| κ-opioid | 1000 (Guinea pig receptor) | >10,000 |
| σ1 | 5000 (Guinea pig receptor) | >10,000 |
| σ2 | - | >10,000 |
Pharmacokinetics
The absorption of clozapine is almost complete, but the oral bioavailability is only 60 to 70% due to first-pass metabolism. The time to peak concentration after oral dosing is about 2.5 hours, and food does not appear to affect the bioavailability of clozapine. The elimination half-life of clozapine is about 14 hours at steady state conditions (varying with daily dose).
Clozapine is extensively metabolized in the liver, via the cytochrome P450 system, to polar metabolites suitable for elimination in the urine and feces. The major metabolite, norclozapine (desmethyl-clozapine), is pharmacologically active. The cytochrome P450 isoenzyme 1A2 is primarily responsible for clozapine metabolism, but 2C, 2D6, 2E1 and 3A3/4 appear to play roles as well. Agents that induce (e.g., cigarette smoke) or inhibit (e.g., theophylline, ciprofloxacin, fluvoxamine) CYP1A2 may increase or decrease, respectively, the metabolism of clozapine. For example, the induction of metabolism caused by smoking means that smokers require up to double the dose of clozapine compared with non-smokers to achieve an equivalent plasma concentration.[54]
Clozapine and norclozapine plasma levels may also be monitored, though they show a significant degree of variation and are higher in women and increase with age.[55] Monitoring of plasma levels of clozapine and norclozapine has been shown to be useful in assessment of compliance, metabolic status, prevention of toxicity, and in dose optimization.[54]
History
Clozapine was synthesized in 1958 by Wander AG, a Swiss pharmaceutical company, based on the chemical structure of the tricyclic antidepressant imipramine. The first test in humans in 1962 was considered a failure. Trials in Germany in 1965 and 1966 as well as a trial in Vienna in 1966 were successful. In 1967 Wander AG was acquired by Sandoz.[6] Further trials took place in 1972 when clozapine was released in Switzerland and Austria as Leponex. Two years later it was released in West Germany, and Finland in 1975. Early testing was performed in the United States around the same time.[56] In 1975, after reports of agranulocytosis leading to death in some clozapine-treated patients, clozapine was voluntarily withdrawn by the manufacturer.[57] Clozapine fell out of favor for more than a decade despite unclear reasons for the agranulocytosis which occurred in Finland, the rate of which was 20 times higher[58] than had been reported in any other country. However, when studies demonstrated that clozapine was more effective against treatment-resistant schizophrenia than other antipsychotics, the FDA and health authorities in most other countries approved its use only for treatment-resistant schizophrenia, and required Restricted Distribution, a Patient Registry and regular hematological monitoring to detect granulocytopenia, before agranulocytosis develops. In December 2002, clozapine was approved in the US for reducing the risk of suicide in schizophrenic or schizoaffective patients judged to be at chronic risk for suicidal behavior.[59] In 2005 FDA approved criteria to allow reduced blood monitoring frequency.[60] In 2015 the individual manufacturer Patient Registries were consolidated by FDA request into a single shared Patient Registry Called The Clozapine REMS Registry.
See also
References
- 1 2 3 4 5 6 7 8 9 "Clozapine". The American Society of Health-System Pharmacists. Retrieved Dec 1, 2015.
- 1 2 Hopfinger A, Esposito EX, Llinas A, Glen RC, Goodman JM (2009). "Findings of the Challenge To Predict Aqueous Solubility". Journal of Chemical Information and Modeling. 49: 1–5. doi:10.1021/ci800436c. PMID 19117422.
- ↑ Essali, A; Al-Haj Haasan, N; Li, C; Rathbone, J (21 January 2009). "Clozapine versus typical neuroleptic medication for schizophrenia.". The Cochrane database of systematic reviews (1): CD000059. doi:10.1002/14651858.CD000059.pub2. PMID 19160174.
- ↑ Siskind, D; McCartney, L; Goldschlager, R; Kisely, S (7 July 2016). "Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis.". The British journal of psychiatry : the journal of mental science. PMID 27388573.
- 1 2 Hartling, L; Abou-Setta, AM; Dursun, S; Mousavi, SS; Pasichnyk, D; Newton, AS (2 October 2012). "Antipsychotics in adults with schizophrenia: comparative effectiveness of first-generation versus second-generation medications: a systematic review and meta-analysis.". Annals of Internal Medicine. 157 (7): 498–511. doi:10.7326/0003-4819-157-7-201210020-00525. PMID 22893011.
- 1 2 Crilly, John (2007-03-01). "The history of clozapine and its emergence in the US market a review and analysis". History of Psychiatry. 18 (1): 39–60. doi:10.1177/0957154X07070335. ISSN 0957-154X. PMID 17580753.
- ↑ Corey, edited by Jie Jack Li, E.J. (2013). Drug discovery practices, processes, and perspectives. Hoboken, N.J.: John Wiley & Sons. p. 248. ISBN 9781118354469.
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ "Clozapine". International Drug Price Indicator Guide. Retrieved 2 December 2015.
- 1 2 Wahlbeck K, Cheine M, Essali MA (2007). Wahlbeck K, ed. "Clozapine versus typical neuroleptic medication for schizophrenia". The Cochrane Database of Systematic Reviews. John Wiley and Sons, Ltd. (2): CD000059. doi:10.1002/14651858.CD000059. PMID 10796289.
- ↑ Meltzer HY (1997). "Treatment-resistant schizophrenia--the role of clozapine". Current Medical Research and Opinion. 14 (1): 1–20. doi:10.1185/03007999709113338. PMID 9524789.
- ↑ Lee M, Dickson RA, Campbell M, Oliphant J, Gretton H, Dalby JT (1998). "Clozapine and substance abuse in patients with schizophrenia". Canadian Journal of Psychiatry. 43: 855–856.
- ↑ "How would you treat someone who has both schizophrenia and Parkinson's disease?".
- ↑ "Clozapine".
- ↑ http://www.clinicalpharmacology-ip.com/Forms/Monograph/monograph.aspx?cpnum=142&sec=monadve
- ↑ "Clozapine".
- ↑ Baggaley M (Apr 2008). "Sexual dysfunction in schizophrenia: focus on recent evidence". Human Psychopharmacology. 23 (3): 201–209. doi:10.1002/hup.924. PMID 18338766.
- ↑ Nielsen J, Correll CU, Manu P, Kane JM (Jun 2013). "Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided?". The Journal of Clinical Psychiatry. 74 (6): 603–13. doi:10.4088/JCP.12r08064. PMID 23842012.
- ↑ Baldessarini, Ross J.; Frank I. Tarazi (2006). "Pharmacotherapy of Psychosis and Maa". In Laurence Brunton; John Lazo; Keith Parker. Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill. ISBN 978-0-07-142280-2. OCLC 150149056.
- ↑ Alvir JM, Lieberman JA, Safferman AZ, Schwimmer JL, Schaaf JA (Jul 1993). "Clozapine-induced agranulocytosis. Incidence and risk factors in the United States". The New England Journal of Medicine. 329 (3): 162–7. doi:10.1056/NEJM199307153290303. PMID 8515788. Free full text with registration
- ↑ Midbari Y, Ebert T, Kosov I, Kotler M, Weizman A, Ram A (Oct 2013). "Hematological and cardiometabolic safety of clozapine in the treatment of very early onset schizophrenia: a retrospective chart review". Journal of Child and Adolescent Psychopharmacology. 23 (8): 516–21. doi:10.1089/cap.2013.0050. PMID 24111981.
- ↑ Haas SJ, Hill R, Krum H, Liew D, Tonkin A, Demos L, Stephan K, McNeil J (2007). "Clozapine-associated myocarditis: a review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993-2003". Drug Safety. 30 (1): 47–57. doi:10.2165/00002018-200730010-00005. PMID 17194170.
- ↑ Ronaldson KJ, Fitzgerald PB, Taylor AJ, Topliss DJ, McNeil JJ (Jun 2011). "A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls". The Australian and New Zealand Journal of Psychiatry. 45 (6): 458–465. doi:10.3109/00048674.2011.572852. PMID 21524186.
- ↑ Ronaldson KJ, Fitzgerald PB, Taylor AJ, Topliss DJ, Wolfe R, McNeil JJ (Nov 2012). "Rapid clozapine dose titration and concomitant sodium valproate increase the risk of myocarditis with clozapine: a case-control study". Schizophrenia Research. 141 (2-3): 173–8. doi:10.1016/j.schres.2012.08.018. PMID 23010488.
- ↑ Every-Palmer S, Nowitz M, Stanley J, Grant E, Huthwaite M, Dunn H, Ellis, PM (March 2016). "Clozapine-treated patients have marked gastrointestinal hypomotility, the probable basis of life-threatening gastrointestinal complications: a cross sectional study". EBioMedicine. 5: 125–134. doi:10.1016/j.ebiom.2016.02.020.
- ↑ Palmer SE, McLean RM, Ellis PM, Harrison-Woolrych M (May 2008). "Life-threatening clozapine-induced gastrointestinal hypomotility: an analysis of 102 cases". The Journal of Clinical Psychiatry. 69 (5): 759–768. doi:10.4088/JCP.v69n0509. PMID 18452342.
- ↑ Townsend G, Curtis D (2006). "Case report: rapidly fatal bowel ischaemia on clozapine treatment". BMC Psychiatry. 6 (1): 43. doi:10.1186/1471-244X-6-43. PMC 1621059
 . PMID 17052340.
. PMID 17052340. - ↑ http://www.medscape.com/viewarticle/409612_2
- ↑ rxlist.com / Clozapine side effects
- ↑ Raja M (Jul 2011). "Clozapine safety, 35 years later". Current Drug Safety. 6 (3): 164–184. doi:10.2174/157488611797579230. PMID 22122392.
- ↑ Barnes TR, Drake MJ, Paton C (Jan 2012). "Nocturnal enuresis with antipsychotic medication". The British Journal of Psychiatry. 200 (1): 7–9. doi:10.1192/bjp.bp.111.095737. PMID 22215862.
- ↑ Ahmed S, Chengappa KN, Naidu VR, Baker RW, Parepally H, Schooler NR (Sep 1998). "Clozapine withdrawal-emergent dystonias and dyskinesias: a case series". The Journal of Clinical Psychiatry. 59 (9): 472–7. doi:10.4088/JCP.v59n0906. PMID 9771818.
- ↑ Szafrański T, Gmurkowski K (1999). "[Clozapine withdrawal. A review]". Psychiatria Polska. 33 (1): 51–67. PMID 10786215.
- ↑ Wirshing DA, Wirshing WC, Kysar L, Berisford MA, Goldstein D, Pashdag J, Mintz J, Marder SR (Jun 1999). "Novel antipsychotics: comparison of weight gain liabilities". The Journal of Clinical Psychiatry. 60 (6): 358–63. doi:10.4088/JCP.v60n0602. PMID 10401912.
- ↑ Nasrallah HA (Jan 2008). "Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles". Molecular Psychiatry. 13 (1): 27–35. doi:10.1038/sj.mp.4002066. PMID 17848919.
- ↑ Albaugh VL, Vary TC, Ilkayeva O, Wenner BR, Maresca KP, Joyal JL, Breazeale S, Elich TD, Lang CH, Lynch CJ (Jan 2012). "Atypical antipsychotics rapidly and inappropriately switch peripheral fuel utilization to lipids, impairing metabolic flexibility in rodents". Schizophrenia Bulletin. 38 (1): 153–166. doi:10.1093/schbul/sbq053. PMC 3245588
 . PMID 20494946.
. PMID 20494946. - ↑ Sproule BA, Naranjo CA, Brenmer KE, Hassan PC (Dec 1997). "Selective serotonin reuptake inhibitors and CNS drug interactions. A critical review of the evidence". Clinical Pharmacokinetics. 33 (6): 454–71. doi:10.2165/00003088-199733060-00004. PMID 9435993.
- ↑ Tiihonen J, Vartiainen H, Hakola P (Jan 1995). "Carbamazepine-induced changes in plasma levels of neuroleptics". Pharmacopsychiatry. 28 (1): 26–28. doi:10.1055/s-2007-979584. PMID 7746842.
- ↑ Besag, FM; Berry, D (2006). "Interactions between antiepileptic and antipsychotic drug". Drug Saf. 29 (2): 95–118. doi:10.2165/00002018-200629020-00001.
- ↑ Jerling, M; Lindstrom, L; Bondesson, U; et al. (1994). ", "Fluvoxamine Inhibition and Carbamazepine Induction of the Metabolism of Clozapine: Evidence From a Therapeutic Drug Monitoring Service". Ther Drug Monit. 16 (4): 368–74. doi:10.1097/00007691-199408000-00006.
- ↑ Bitter R, Demler TL, Opler L (Sep 2008). "Safety evaluation of the concomitant use of clozapine and benzodiazepines: a retrospective, cross-sectional chart review". Journal of Psychiatric Practice. 14 (5): 265–70. doi:10.1097/01.pra.0000336753.11943.7c. PMID 18832957.
- ↑ Raaska K, Neuvonen PJ (Nov 2000). "Ciprofloxacin increases serum clozapine and N-desmethylclozapine: a study in patients with schizophrenia". European Journal of Clinical Pharmacology. 56 (8): 585–9. doi:10.1007/s002280000192. PMID 11151749.
- ↑ Prescribing information. Clozaril (clozapine). East Hanover, NJ: Novartis Pharmaceuticals Corporation, September 2014.
- ↑ Novartis Pharmaceuticals (April 2006). "Prescribing Information" (PDF). Novartis Pharmaceuticals. p. 36. Retrieved 2007-06-29.
- ↑ Naheed M, Green B (2001). "Focus on clozapine". Current Medical Research and Opinion. 17 (3): 223–9. doi:10.1185/0300799039117069. PMID 11900316.
- ↑ http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=2818
- ↑ Robinson DS (2007). "CNS Receptor Partial Agonists: A New Approach to Drug Discovery". Primary Psychiatry. 14 (8): 22–24.
- ↑ Wu Y, Blichowski M, Daskalakis ZJ, Wu Z, Liu CC, Cortez MA, Snead OC (Sep 2011). "Evidence that clozapine directly interacts on the GABAB receptor". NeuroReport. 22 (13): 637–41. doi:10.1097/WNR.0b013e328349739b. PMID 21753741.
- ↑ Vacher CM, Gassmann M, Desrayaud S, Challet E, Bradaia A, Hoyer D, Waldmeier P, Kaupmann K, Pévet P, Bettler B (May 2006). "Hyperdopaminergia and altered locomotor activity in GABAB1-deficient mice". Journal of Neurochemistry. 97 (4): 979–91. doi:10.1111/j.1471-4159.2006.03806.x. PMID 16606363.
- ↑ Wierońska JM, Kusek M, Tokarski K, Wabno J, Froestl W, Pilc A (Jul 2011). "The GABA B receptor agonist CGP44532 and the positive modulator GS39783 reverse some behavioural changes related to positive syndromes of psychosis in mice". British Journal of Pharmacology. 163 (5): 1034–47. doi:10.1111/j.1476-5381.2011.01301.x. PMC 3130949
 . PMID 21371011.
. PMID 21371011. - ↑ Tanahashi S, Yamamura S, Nakagawa M, Motomura E, Okada M (Mar 2012). "Clozapine, but not haloperidol, enhances glial D-serine and L-glutamate release in rat frontal cortex and primary cultured astrocytes". British Journal of Pharmacology. 165 (5): 1543–55. doi:10.1111/j.1476-5381.2011.01638.x. PMC 3372736
 . PMID 21880034.
. PMID 21880034. - ↑ Xi D, Li YC, Snyder MA, Gao RY, Adelman AE, Zhang W, Shumsky JS, Gao WJ (May 2011). "Group II metabotropic glutamate receptor agonist ameliorates MK801-induced dysfunction of NMDA receptors via the Akt/GSK-3β pathway in adult rat prefrontal cortex". Neuropsychopharmacology. 36 (6): 1260–74. doi:10.1038/npp.2011.12. PMC 3079418
 . PMID 21326193.
. PMID 21326193. - ↑ Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 2013-10-10 from "Archived copy". Archived from the original on 2013-11-08. Retrieved 2013-11-25..
- 1 2 Rostami-Hodjegan A, Amin AM, Spencer EP, Lennard MS, Tucker GT, Flanagan RJ (Feb 2004). "Influence of dose, cigarette smoking, age, sex, and metabolic activity on plasma clozapine concentrations: a predictive model and nomograms to aid clozapine dose adjustment and to assess compliance in individual patients". Journal of Clinical Psychopharmacology. 24 (1): 70–8. doi:10.1097/01.jcp.0000106221.36344.4d. PMID 14709950.
- ↑ Lane HY, Chang YC, Chang WH, Lin SK, Tseng YT, Jann MW (Jan 1999). "Effects of gender and age on plasma levels of clozapine and its metabolites: analyzed by critical statistics". The Journal of Clinical Psychiatry. 60 (1): 36–40. doi:10.4088/JCP.v60n0108. PMID 10074876.
- ↑ Crilly J (Mar 2007). "The history of clozapine and its emergence in the US market: a review and analysis". History of Psychiatry. 18 (1): 39–60. doi:10.1177/0957154X07070335. PMID 17580753.
- ↑ Healy, David (2004). The Creation of Psychopharmacology. Cambridge: Harvard University Press. pp. 238–42. ISBN 0-674-01599-1.
- ↑ Griffith, R.W.; Saameli, K. "CLOZAPINE AND AGRANULOCYTOSIS". The Lancet. 306 (7936). doi:10.1016/s0140-6736(75)90135-x.
- ↑ "Supplemental NDA Approval Letter for Clozaril, NDA 19-758 / S-047" (PDF). United States Food and Drug Administration. December 18, 2002. Archived (PDF) from the original on November 23, 1012. Retrieved November 23, 2012.
- ↑ http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2005/019758s054ltr.pdf
Further reading
- Benkert, Hippius: Kompendium der Psychiatrischen Pharmakotherapie (German), 4th. ed., Springer Verlag
- B. Bandelow, S. Bleich, and S. Kropp: Handbuch Psychopharmaka (German), 2nd. ed. Hogrefe
- Crilly J (Mar 2007). "The history of clozapine and its emergence in the US market: a review and analysis". History of Psychiatry. 18 (1): 39–60. doi:10.1177/0957154X07070335. PMID 17580753.