Levocetirizine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Xyzal, Levazyr |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a607056 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | R06AE09 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | High |
| Protein binding | 90% |
| Metabolism | Hepatic 14% CYP3A4 |
| Biological half-life | 6 to 10 hours |
| Excretion | Renal and fecal |
| Identifiers | |
| |
| CAS Number |
130018-77-8 |
| PubChem (CID) | 1549000 |
| IUPHAR/BPS | 1214 |
| ChemSpider |
1266001 |
| UNII |
6U5EA9RT2O |
| KEGG |
D07402 |
| ChEMBL |
CHEMBL1201191 |
| Chemical and physical data | |
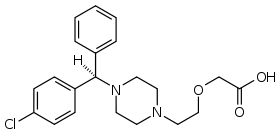
| Formula | C21H25ClN2O3 |
| Molar mass | 388.888 g/mol |
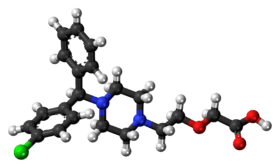
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Levocetirizine (as levocetirizine dihydrochloride) is a third-generation non-sedative antihistamine, developed from the second-generation antihistamine cetirizine. Chemically, levocetirizine is the active enantiomer of cetirizine. It is the R-enantiomer of the cetirizine racemate. Levocetirizine is an inverse agonist that decreases activity at histamine H1 receptors. This in turn prevents the release of other allergy chemicals and increased blood supply to the area, and provides relief from the typical symptoms of hay fever. It does not prevent the actual release of histamine from mast cells.
The manufacturers claim it to be more effective with fewer side effects than the second-generation drugs; however, there have been no published studies supporting this assertion. A study part-funded by the manufacturer UCB concluded it may be more effective than some other second- and third-generation anti-histamines, but didn't compare it to cetirizine.[1]
History
Levocetirizine was first launched in 2001 by Belgian pharmaceutical company UCB.
Brand names
It is sold under the brand names:
- Xyzal /ˈzaɪzæl/ in Australia, Austria, Croatia, Czech Republic, Finland, France, India, Ireland (also Rinozal), Lithuania, Netherlands, Portugal, Romania, Taiwan, Turkey, The Philippines, Serbia, Slovakia, Slovenia, South Africa and UK. On May 25, 2007, the United States Food and Drug Administration approved Xyzal, where it is co-marketed by Sanofi-Aventis.
- Xusal in Germany;
- Xozal in Greece;
- Cezera in Poland;
- Xazal in Spain;
- Xuzal in Mexico;
- In Hungary it is marketed as Zilola (made by Richter Gedeon) and Histisynt (Actavis).
- In India, levocetirizine is marketed by GlaxoSmithKline under the brand name Vozet. Torrent Pharma launched UVNIL for the rural market.
- In Pakistan levocetirizine was first launched in a liquid formulation by Novartis Consumer Health Division under the name of T-Day Syrup.
- In Nepal levocetirizine is available in tablet with brand name Curin manufactured by Beximco Pharma.[2]
- In Chile, it is marketed as Zival and made by Laboratorio Saval.
- In Sweden, Xyzal was not granted subventions, and was withdrawn from the market.[3]
Side effects
Levocetirizine is called a non-sedating antihistamine as it does not enter the brain in significant amounts, and is therefore unlikely to cause drowsiness. However, some people may experience some slight sleepiness, headache, mouth dryness, lightheadedness, vision problems (mainly blurred vision), palpitations and fatigue.[4]
Research
Latest research shows levocetirizine reduces asthma attacks by 70% in children.[5]
Availability
The drug is currently available by prescription in the United States. Although the drug was only authorized by the FDA on 25 May 2007, it was already available in most European countries. Like many new drugs it entered the market at a higher price than currently available third and second generation antihistamines. In India, one form of the drug is available as Crohist MK tablets and syrup, a formulation of levocetirizine hydrochloride and montelukast. In India, Crohist MK is a Schedule 'H' drug and may only be prescribed by a registered medical practitioner. In Finland, Hungary, China, and Kuwait[6] the drug is sold over-the-counter.
References
- ↑ Grant, JA; Riethuisen, JM; Moulaert, B; DeVos, C; Gamalero, C.; Descalzi, D.; Folli, C.; Passalacqua, G.; Canonica, G.W. (February 2002). "A double-blind, randomized, single-dose, crossover comparison of levocetirizine with ebastine, fexofenadine, loratadine, mizolastine, and placebo: suppression of histamine-induced wheal-and-flare response during 24 hours in healthy male subjects". Ann Allergy Asthma Immunol. 88 (2): 190–197. doi:10.1016/S1081-1206(10)61995-3. PMID 11868924.
- ↑ https://web.archive.org/web/20121022184859/http://beximco-pharma.com/allergic-disorders/147-curin.html. Archived from the original on October 22, 2012. Retrieved August 29, 2012. Missing or empty
|title=(help) - ↑ http://www.fass.se/LIF/substance?substanceId=ID11EHWRUFGBA53CSR
- ↑ XOZAL technical specifications booklet.
- ↑ Pasquali, M; Baiardini, I; Rogkakou, A; Riccio, AM; Gamalero, C; Descalzi, D; Folli, C; Passalacqua, G; Canonica, GW (September 2006). "Levocetirizine in persistent allergic rhinitis and asthma: effects on symptoms, quality of life and inflammatory parameters". Clinical & Experimental Allergy. 36 (9): 1161–7. doi:10.1111/j.1365-2222.2006.02548.x. PMID 16961716.
- ↑ On different names: http://www.hazipatika.com/gyogyszerkereso/termek/sefaller_5_mg_filmtabletta/54120