Phorate
 | |
| Names | |
|---|---|
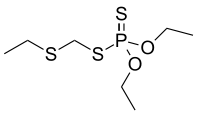
| IUPAC name
O,O-Diethyl S-[(ethylsulfanyl)methyl] phosphorodithioate | |
| Other names
Thimet (trademark) | |
| Identifiers | |
| 298-02-2 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:38764 |
| ChEMBL | ChEMBL510014 |
| ChemSpider | 4626 |
| ECHA InfoCard | 100.005.503 |
| PubChem | 4790 |
| |
| |
| Properties | |
| C7H17O2PS3 | |
| Molar mass | 260.36 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Skunk-like[1] |
| Density | 1.16 g/mL |
| Melting point | −43 °C; −45 °F; 230 K [1] |
| Boiling point | 118-120°C (2.0 mm Hg)[2] |
| 0.005% (20°C)[1] | |
| Vapor pressure | 0.0008 mmHg (20°C)[1] |
| Hazards | |
| Flash point | 160 °C; 320 °F; 433 K (open cup)[1] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
none[1] |
| REL (Recommended) |
TWA 0.05 mg/m3 ST 0.2 mg/m3 [skin][1] |
| IDLH (Immediate danger) |
N.D.[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Phorate is an organophosphate used as an insecticide and acaricide.
Overview
At normal conditions, it is a pale yellow mobile liquid poorly soluble in water but readily soluble in organic solvents. It is relatively stable and hydrolyses only at very acidic or basic conditions. It is very toxic both for target organisms and for mammals including human. It inhibits acetylcholinesterase and butyrylcholinesterase.[3]
Phorate is most commonly applied in granular form. It is non-biocumulative and has no residual action. But some metabolites may persist in soil. It also damages some seeds.[3]
Toxicity
Phorate is absorbed readily through all ways. Its toxicity is high. Oral LD50 to rats is 1.1 – 3.2 mg/kg, to mice 3.5 – 6.5 mg/kg (technical phorate). Similar values has been found out to birds.[3]
References
- 1 2 3 4 5 6 7 8 "NIOSH Pocket Guide to Chemical Hazards #0502". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Farm Chemicals Handbook, Meister Publishing Co., Willoughby, OH (1991)
- 1 2 3 Data sheets on pesticides No. 75 – Phorate
External links
- Phorate in the Pesticide Properties DataBase (PPDB)