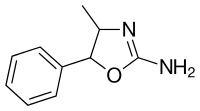
4-Methylaminorex
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral, Vaporized, Insufflated, Injected |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 62% oral; 79% nasal; 91 - 93.5% smoked; 100% IV |
| Metabolism | Hepatic |
| Biological half-life | 10-19 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
3568-94-3 29493-77-4 - (±)-cis isomers |
| PubChem (CID) | 92196 |
| DrugBank |
DB01447 |
| ChemSpider |
83237 |
| Chemical and physical data | |
| Formula | C10H12N2O |
| Molar mass | 176.21 |
| 3D model (Jmol) | Interactive image |
| Chirality | Racemic mixture |
| |
| |
| | |
4-Methylaminorex (4-MAR, 4-MAX) is a stimulant drug of the 2-amino-5-aryloxazoline class that was first synthesized in 1960 by McNeil Laboratories.[1] It is also known by its street names "U4Euh" ("Euphoria") and "Ice". It is banned in many countries as a stimulant.
4-Methylaminorex has effects comparable to methamphetamine but with a longer duration.
The results of animal experiments conducted with this drug suggest that it has an abuse liability similar to cocaine and amphetamine. One study found that, "stimulus properties of racemic cis, racemic trans, and all four individual optical isomers of 4-methylaminorex were examined in rats trained to discriminate 1 mg/kg of S(+)amphetamine sulfate from saline. The S(+)amphetamine stimulus generalized to all of the agents investigated".[2] A second study in which rats trained to discriminate either 0.75 mg/kg S(+)-amphetamine or 1.5 mg/kg fenfluramine from saline generalized to aminorex as amphetamine stimulus but not to fenfluramine.[3] Rats trained to discriminate 8 mg/kg cocaine from saline generalized 4-methylaminorex to cocaine-stimulus.[4] The reinforcing effects of cis-4-methylaminorex were determined in two models of intravenous drug self-administration in primates. Vehicle or 4-methylaminorex doses were substituted for cocaine. One of the two different doses of 4-methylaminorex maintained self-administration behavior above vehicle control levels.[5]
Chemistry
4-Methylaminorex exists as four stereoisomers - (±)-cis and (±)-trans. The (±)-cis isomers are the form used recreationally. The (±)-cis isomers [racemate (1:1-mixture) of the (4R,5S)-isomer and the enantiomeric (4S,5R)-isomer] generally synthesized from dl-phenylpropanolamine in one step by cyclization with cyanogen bromide (sometimes prepared in situ by reacting sodium cyanide with bromine). Alternate synthesis routes generally involve more steps, such as replacing cyanogen bromide with sodium or potassium cyanate to form an intermediate and then reacting it with concentrated hydrochloric acid. A method reported in microgram replaced the need for a separate addition of hydrochloric acid by starting with the hydrochloride salt of the dl-phenylpropanolamine but side-products are noted. The (±)-trans isomers [racemate (1:1-mixture) of the (4S,5S)-isomer and the enantiomeric (4R,5R)-isomer] are synthesized in the same manner above but dl-norephedrine is used as the starting material instead. The cyanate reaction proceeds differently from the cyanogen bromide and transforms norephedrine into trans-4-methylaminorex instead, as noted in the DEA micrograph. The cyanogen bromide, by comparison, transformed norephedrine into the cis isomer and norpseudoephedrine into the trans isomers of the final product.
Dosage
4-methylaminorex can be smoked, insufflated or taken orally.
As an anorectic, the ED50 is 8.8 mg/kg in rats for the (±)-cis isomers. The (±)-trans isomers are slightly more potent at 7.0 mg/kg. As a recreational drug, the effective dosage ranges from 5 to 25 mg.[6]
In the 1970s McNeil Laboratories, Inc. was trying to bring 4-methylaminorex to drug market as a sympathomimetic (most commonly used as asthma-medicines), research name was McN-822, they mention that human dose would have been 0.25 mg/kg of body weight. They mention also LD50: 17 mg/kg p.o for mice [7]
There is a patent about the use of 4-methylaminorex "as a nasal decongestant which, when administered orally, does not produce adverse central nervous system stimulant effects as experienced with other decongestants and anorexiants." Dose mentioned is 0.25 mg/kg of body weight.[8]
Effects
It produces long-lasting effects, generally up to 16 hours in duration if taken orally and up to 12 hours if smoked or insufflated. Large doses have been reported anecdotally to last up to 36 hours. The effects are stimulant in nature, producing euphoria, an increase in attention, and increased cognition. Anecdotally, it has been reported to produce effects similar to nootropics, however, there is no research to support the claim that it is any different or more effective than other psychostimulants in this respect. Moreover, 4-methylaminorex does not have the established safety profile of widely used clinical psychostimulants such as methylphenidate and dextroamphetamine.
| Time (h) | Concentration of 4-methylaminorex in urine (µg/ml) |
|---|---|
| 0-6 | 45 |
| 6-24 | 1.0 |
| 24-36 | 0.1 |
| 36-48 | not detected |
There has been one reported death due to 4-methylaminorex and diazepam. Concentrations of 4-methylaminorex were: in blood 21.3 mg/L; in urine 12.3 mg/L. Diazepam concentration in blood was 0.8 mg/L.[9] One rat study[10] has studied excretion of 4-methylaminorex in urine: "The concentration of trans-4-methylaminorex in rat urine following four injections of the trans-4S,5S isomer (5 mg/kg i.p each, at intervals of 12 h in 2 days, as measured quantitatively by GC/MS"
Also another study has studied pharmacokinetics and tissue distribution of the stereoisomers of 4-methylaminorex in rats.[11]
"Pulmonary hypertension has been associated with ingestion of the appetite suppressant aminorex. A similar compound, 4-methylaminorex was discovered on the property of three individuals with diagnoses of pulmonary hypertension."[12]
Neurotoxicity studies
There have been three studies studying possible neurotoxicity of 4-methylaminorex. First study[13] using quite high doses (highest dose caused clonic seizures and some rats died) in rats and studying short-term effects (rats were killed 30 min to 18 h after injection of 5, 10 or 20 mg/kg of racemic cis-4-methylaminorex) suggested reduction in tryptophan hydroxylase (TPH) activity (a possible marker for serotonin neurotoxicity) but citing study: "No change in TPH activity was observed 30 min after injection; by 8 h the activity of this enzyme appeared to be recovering." and "this agent is significantly less neurotoxic than methamphetamine or MDMA."
Study[14] published 2 years later than first one also suggested reduction in tryptophan hydroxylase activity, they used quite high dose too (10 mg/kg of cis-4-methylaminorex) and studied also long-term effects (rats were killed 3 h, 18 h or 7 days after injection), they found reduction of 20-40% of tryptophan hydroxylase (TPH) activity and "recovery of TPH activity occurred 18 h after treatment, but was significantly decreased again by 7 days." but "It is noteworthy that, unlike the other analogs, the striatal levels of 5-HT did not decline with TPH activity following multiple 4-methylaminorex treatment"
Latest study[15] (using mice) was not able to find any long-term effects suggesting neurotoxicity and they found instead increase in serotonin levels, they also used high doses (15 mg/kg of each isomers studied) "The dosages used in the present experiments are about 6-10 times than the effective doses of aminorex and stereoisomers inhibition of food intake." Doses were repeated 3 times a day and mice were killed 7 days after last dose. "Since a long-lasting depletion of dopamine or 5-HT appears to be a good predictor of dopamine or 5-HT neurotoxicity (Wagner et al. 1980; Ricaurte et al. 1985), the results suggest that the aminorex compounds except 4S,SS-dimethylaminorex, unlike MDMA or fenfluramine, are not toxic to either dopamine or 5-HT neurotransmitter systems in CBA mice. It was reported that although multiple doses of 4-methylaminorex caused long-term (7 days) declines in striatal tryptophan hydroxylase activity in SD rats, no changes were found in 5-HT and 5-HIAA levels (Hanson et al. 1992).[12]
That first study [11] also suggested reduced dopamine (DA) levels (a possible marker for dopamine neurotoxicity), but citing study: "However, 8 h after drug administration no differences from control values were seen in DA, DOPAC or HVA levels." and again later studies [12-13] didn't find any long-term reduction.
Legal status
Australia
In Australia, 4-Methylaminorex is listed as Schedule 9, making it legal only for scientific and medical research.[16]
Canada
In Canada, 4-Methylaminorex is listed as Schedule III.[17]
Netherlands
In the Netherlands, aminorex (4-methylaminorex is a designer drug 2014) is a List I drug of the Opium Law.[18] It is not approved by the CBG, and so it is designated as lacking any medical use.
United Kingdom
In the United Kingdom, 4-Methylaminorex is listed as Class A.[19]
United States
In the United States, (±)-cis-4-methylaminorex was placed in Schedule I of the Controlled Substances Act shortly after its emergence as a recreational drug in the mid-1980s.[20] Manufacturing the trans isomer required a different process than those encountered when the substance was first scheduled, and was believed less potent than the cis isomer with a much lower abuse potential. However, studies revealing the abuse potential of the 'trans' isomer, coupled with the development of new clandestine synthetic methods that would produce the trans created a potential loophole in the law, which covered only the 'cis' isomer. To clarify the situation, the US Drug Enforcement Administration published a paper in its DEA Microgram Journal, regarding interpretation of the relevant statutory law as it relates to the status of trans-4-methylaminorex. In summary, according to this non-legally binding decision, trans-4-methylaminorex is not currently a controlled substance, but a potential analogue. In fact, the report explicitly states:
The United States [Drug Enforcement Administration] has the following opinion on the legality of the positional isomer "trans"-4-methylaminorex, which, unlike its 'cis' isomer was never placed in any schedule under the Controlled Substances Act.
However, the opinion does say that the agency considers the substance a potential controlled substance analogue, making the substance identical to a Schedule I substance if intended for human consumption, according to the Federal Analogue Act. The report gives an account of a successful conviction under the Federal Analogue Act of an offense involving the trans isomer.[21]
Florida
"2-Amino-4-methyl-5-phenyl-2-oxazoline (4-methylaminorex)" and "any material, compound, mixture, or preparation that contains any quantity of" it "or that contains any of [its] salts, isomers, including optical, positional, or geometric isomers, and salts of isomers, if the existence of such salts, isomers, and salts of isomers is possible" is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess in Florida.[22]
See also
External links
References
- ↑ US Patent 3278382 - 2-amino-5-aryloxazoline compositions and methods of using same
- ↑ Glennon RA, Misenheimer B (March 1990). "Stimulus properties of a new designer drug: 4-methylaminorex ("U4Euh")". Pharmacol. Biochem. Behav. 35 (3): 517–21. doi:10.1016/0091-3057(90)90282-M. PMID 1971111.
- ↑ Young R (May 1992). "Aminorex produces stimulus effects similar to amphetamine and unlike those of fenfluramine". Pharmacol. Biochem. Behav. 42 (1): 175–8. doi:10.1016/0091-3057(92)90462-O. PMID 1356272.
- ↑ Young R, Glennon RA (May 1993). "Cocaine-stimulus generalization to two new designer drugs: methcathinone and 4-methylaminorex". Pharmacol. Biochem. Behav. 45 (1): 229–31. doi:10.1016/0091-3057(93)90110-F. PMID 8516363.
- ↑ Mansbach RS, Sannerud CA, Griffiths RR, Balster RL, Harris LS (October 1990). "Intravenous self-administration of 4-methylaminorex in primates". Drug Alcohol Depend. 26 (2): 137–44. doi:10.1016/0376-8716(90)90120-4. PMID 2242714.
- ↑ "Erowid 4-methylaminorex Vault : Dosage".
- ↑ "System Timed Out (Library of Congress Online Catalog)".
- ↑ "Method of decongesting the nose ... - Google Patents".
- ↑ Davis FT, Brewster ME (March 1988). "A fatality involving U4Euh, a cyclic derivative of phenylpropanolamine". J. Forensic Sci. 33 (2): 549–53. PMID 3373171.
- ↑ Kankaanpää A, Meririnne E, Ellermaa S, Ariniemi K, Seppälä T (September 2001). "Detection and assay of cis- and trans-isomers of 4-methylaminorex in urine, plasma and tissue samples". Forensic Sci. Int. 121 (1-2): 57–64. doi:10.1016/S0379-0738(01)00453-4. PMID 11516888.
- ↑ Meririnne E, Ellermaa S, Kankaanpää A, Bardy A, Seppälä T (June 2004). "Pharmacokinetics and tissue distribution of the stereoisomers of 4-methylaminorex in the rat". J. Pharmacol. Exp. Ther. 309 (3): 1198–205. doi:10.1124/jpet.103.060053. PMID 14742748.
- 1 2 Gaine SP, Rubin LJ, Kmetzo JJ, Palevsky HI, Traill TA (November 2000). "Recreational use of aminorex and pulmonary hypertension". Chest. 118 (5): 1496–7. doi:10.1378/chest.118.5.1496. PMID 11083709.
- ↑ Bunker CF, Johnson M, Gibb JW, Bush LG, Hanson GR (May 1990). "Neurochemical effects of an acute treatment with 4-methylaminorex: a new stimulant of abuse". Eur. J. Pharmacol. 180 (1): 103–11. doi:10.1016/0014-2999(90)90597-Y. PMID 1973111.
- ↑ Hanson GR, Bunker CF, Johnson M, Bush L, Gibb JW (August 1992). "Response of monoaminergic and neuropeptide systems to 4-methylaminorex: a new stimulant of abuse". Eur. J. Pharmacol. 218 (2-3): 287–93. doi:10.1016/0014-2999(92)90181-3. PMID 1358636.
- ↑ Zheng Y, Russell B, Schmierer D, Laverty R (January 1997). "The effects of aminorex and related compounds on brain monoamines and metabolites in CBA mice". J. Pharm. Pharmacol. 49 (1): 89–96. doi:10.1111/j.2042-7158.1997.tb06758.x. PMID 9120777.
- ↑ "Poisons Standard 2009". Retrieved 2009-09-02.
- ↑ "Controlled Drugs and Substances Act". Retrieved 2009-09-02.
- ↑ "Bijlage 1 Lijst I Opiumwetmiddelen". Retrieved 2009-09-02.
- ↑ "LIST OF DRUGS CURRENTLY CONTROLLED UNDER CLASS A" (PDF). Retrieved 2009-09-02.
- ↑ "Section 1308.11 Schedule I". Archived from the original on 27 August 2009. Retrieved 2009-09-02.
- ↑ Synthesis of trans-4-Methylaminorex from Norephedrine and Potassium Cyanate (DEA Microgram Journal)
- ↑ Florida Statutes - Chapter 893 - DRUG ABUSE PREVENTION AND CONTROL