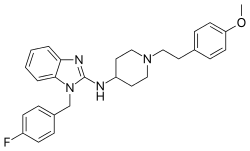
Astemizole
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Multum Consumer Information |
| MedlinePlus | a600034 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | R06AX11 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | ~96% |
| Metabolism | Hepatic (CYP3A4)[1] |
| Biological half-life | 24 hours |
| Excretion | Fecal |
| Identifiers | |
| |
| CAS Number |
68844-77-9 |
| PubChem (CID) | 2247 |
| IUPHAR/BPS | 2603 |
| DrugBank |
DB00637 |
| ChemSpider |
2160 |
| UNII |
7HU6337315 |
| KEGG |
D00234 |
| ChEBI |
CHEBI:2896 |
| ChEMBL |
CHEMBL296419 |
| Chemical and physical data | |
| Formula | C28H31FN4O |
| Molar mass | 458.571 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Astemizole (marketed under the brand name Hismanal, developmental code R43512) was a second-generation antihistamine drug that has a long duration of action. Astemizole was discovered by Janssen Pharmaceutica in 1977. It has been withdrawn from the market in most countries because of rare but potentially fatal side effects (QTc interval prolongation and related arrhythmias due to hERG channel blockade).[2]
Pharmacology
Astemizole is a histamine H1-receptor antagonist. It has anticholinergic and antipruritic effects.
Astemizole is rapidly absorbed from the gastrointestinal tract and competitively binds to histamine H1 receptor sites in the gastrointestinal tract, uterus, blood vessels, and bronchial muscle. This suppresses the formation of edema and pruritus (caused by histamine).
Despite some earlier reports that astemizole does not cross the blood–brain barrier, several studies[3][4] have shown high permeability and high binding to protein folds associated with Alzheimer's.
Astemizole may also act on histamine H3 receptors, thereby producing adverse effects.
Astemizole does also act as FIASMA (functional inhibitor of acid sphingomyelinase).[5]
Toxicity
Astemizole has an oral LD50 of approximately 2052 mg/kg (in mice).
References
- ↑ Matsumoto S, Yamazoe Y (February 2001). "Involvement of multiple human cytochromes P450 in the liver microsomal metabolism of astemizole and a comparison with terfenadine". British Journal of Clinical Pharmacology. 51 (2): 133–42. doi:10.1046/j.1365-2125.2001.01292.x. PMC 2014443
 . PMID 11259984.
. PMID 11259984. - ↑ Zhou Z, Vorperian VR, Gong Q, Zhang S, January CT (June 1999). "Block of HERG potassium channels by the antihistamine astemizole and its metabolites desmethylastemizole and norastemizole". J. Cardiovasc. Electrophysiol. 10 (6): 836–43. doi:10.1111/j.1540-8167.1999.tb00264.x. PMID 10376921.
- ↑ Rojo, Leonel E.; Alzate-Morales, Jans; Saavedra, Iván N.; Davies, Peter; Maccioni, Ricardo B. (2010). "Selective Interaction of Lansoprazole and Astemizole with Tau Polymers: Potential New Clinical Use in Diagnosis of Alzheimer's Disease". Journal of Alzheimer's Disease. IOS Press. 19 (2): 573–589. doi:10.3233/JAD-2010-1262. ISSN 1875-8908. PMC 2951486
 . PMID 20110603.
. PMID 20110603. - ↑ Di, Li; Kerns, Edward H; Fan, Kristi; McConnell, Oliver J; Carter, Guy T (7 March 2003). "High throughput artificial membrane permeability assay for blood–brain barrier". European Journal of Medicinal Chemistry. 38 (3): 223–232. doi:10.1016/S0223-5234(03)00012-6.
- ↑ Kornhuber J, Muehlbacher M, Trapp S, Pechmann S, Friedl A, Reichel M, Mühle C, Terfloth L, Groemer T, Spitzer G, Liedl K, Gulbins E, Tripal P (2011). "Identification of novel functional inhibitors of acid sphingomyelinase". PLoS ONE. 6 (8): e23852. doi:10.1371/journal.pone.0023852. PMC 3166082
 . PMID 21909365.
. PMID 21909365.