Norketamine
 | |
| Clinical data | |
|---|---|
| ATC code | None |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
35211-10-0 79499-59-5 (HCl) |
| PubChem (CID) | 123767 |
| ChemSpider | 110322 |
| UNII | XQY6JVF94X |
| ChEMBL | CHEMBL1039 |
| Chemical and physical data | |
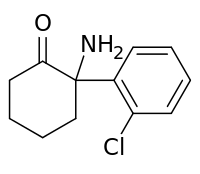
| Formula | C12H14ClNO |
| Molar mass | 223.69866 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Norketamine, or N-desmethylketamine, is the major active metabolite of ketamine, which is formed mainly by CYP3A4.[1][2] Similarly to ketamine, norketamine acts as a noncompetitive NMDA receptor antagonist (Ki = 1.7 µM and 13 µM for (S)-(+)-norketamine and (R)-(–)-norketamine, respectively),[1][3] but is about 3–5 times less potent as an anesthetic in comparison.[2][4] Also, similarly again to ketamine, norketamine binds to the μ- and κ-opioid receptors.[5] Relative to ketamine, norketamine is much more potent as an antagonist of the α7-nicotinic acetylcholine receptor, and produces rapid antidepressant effects in animal models which have been reported to correlate with its activity at this receptor.[6] However, norketamine is about 1/5th as potent as ketamine as an antidepressant in mice as per the forced swim test, and this seems also to be in accordance with its 3–5-fold reduced comparative potency in vivo as an NMDA receptor antagonist.[7] Norketamine is metabolized into dehydronorketamine and hydroxynorketamine, which are far less or negligibly active as NMDA receptor antagonists in comparison[2] but retain activity as potent antagonists of the α7-nicotinic acetylcholine receptor.[8][9]
See also
References
- 1 2 A. P. Adams; J. N. Cashman; R. M. Grounds (12 January 2002). Recent Advances in Anaesthesia and Intensive Care:. Cambridge University Press. pp. 42–. ISBN 978-1-84110-117-0.
- 1 2 3 Donald G. Barceloux (3 February 2012). Medical Toxicology of Drug Abuse: Synthesized Chemicals and Psychoactive Plants. John Wiley & Sons. pp. 112–. ISBN 978-1-118-10605-1.
- ↑ Howard S. Smith (21 December 2008). Current Therapy in Pain. Elsevier Health Sciences. pp. 482–. ISBN 1-4377-1117-0.
- ↑ T.H. Stanley; P.G. Schafer (6 December 2012). Pediatric and Obstetrical Anesthesia: Papers presented at the 40th Annual Postgraduate Course in Anesthesiology, February 1995. Springer Science & Business Media. pp. 372–. ISBN 978-94-011-0319-0.
- ↑ Bradford P. Smith (21 April 2014). Large Animal Internal Medicine. Elsevier Health Sciences. pp. 30–. ISBN 978-0-323-08840-4.
- ↑ Paul, Rajib K.; Singh, Nagendra S.; Khadeer, Mohammed; Moaddel, Ruin; Sanghvi, Mitesh; Green, Carol E.; O’Loughlin, Kathleen; Torjman, Marc C.; Bernier, Michel; Wainer, Irving W. (2014). "(R,S)-Ketamine Metabolites (R,S)-norketamine and (2S,6S)-hydroxynorketamine Increase the Mammalian Target of Rapamycin Function". Anesthesiology. 121 (1): 149–159. doi:10.1097/ALN.0000000000000285. ISSN 0003-3022. PMID 24936922.
- ↑ Sałat K, Siwek A, Starowicz G, Librowski T, Nowak G, Drabik U, et al. (2015). "Antidepressant-like effects of ketamine, norketamine and dehydronorketamine in forced swim test: Role of activity at NMDA receptor". Neuropharmacology. 99: 301–7. doi:10.1016/j.neuropharm.2015.07.037. PMID 26240948.
- ↑ Moaddel, Ruin; Abdrakhmanova, Galia; Kozak, Joanna; Jozwiak, Krzysztof; Toll, Lawrence; Jimenez, Lucita; Rosenberg, Avraham; Tran, Thao; Xiao, Yingxian; Zarate, Carlos A.; Wainer, Irving W. (2013). "Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors". European Journal of Pharmacology. 698 (1-3): 228–234. doi:10.1016/j.ejphar.2012.11.023. ISSN 0014-2999.
- ↑ Robin A.J. Lester (11 November 2014). Nicotinic Receptors. Springer. pp. 445–. ISBN 978-1-4939-1167-7.
| Inhalational | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Injection |
| ||||||||||||||
| |||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| |||||||||||||||||
| Receptor (ligands) |
| ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transporter (blockers) |
| ||||||||||||||||||||||||||||||||||||||||||
| Enzyme (inhibitors) |
| ||||||||||||||||||||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||||||||||||||||||||
See also: GABAergics • GHBergics • Glycinergics | |||||||||||||||||||||||||||||||||||||||||||
| MOR |
|
|---|---|
| DOR |
|
| KOR |
|
| NOP |
|
| Unsorted |
|
| Others |
|
See also: Peptide receptor modulators | |