Salbutamol
 | |
|
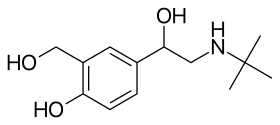
Salbutamol (top), (R)-(−)-salbutamol (center) and (S)-(+)-salbutamol (bottom) | |
| Clinical data | |
|---|---|
| Trade names | Ventolin, Proventil, others[1] |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category | |
| Routes of administration | Oral, inhalational, IV |
| ATC code | R03AC02 (WHO) R03CC02 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Liver |
| Onset of action | <15 min (inhaled), <30 min (pill)[3] |
| Biological half-life | 3.8–6 hours |
| Duration of action | 2–6 hrs[3] |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number |
18559-94-9 |
| PubChem (CID) | 2083 |
| IUPHAR/BPS | 558 |
| DrugBank |
DB01001 |
| ChemSpider |
1999 |
| UNII |
QF8SVZ843E |
| KEGG |
D02147 |
| ChEBI |
CHEBI:2549 |
| ChEMBL |
CHEMBL714 |
| ECHA InfoCard | 100.038.552 |
| Chemical and physical data | |
| Formula | C13H21NO3 |
| Molar mass | 239.311 g/mol |
| 3D model (Jmol) | Interactive image |
| Chirality | Racemic mixture |
| |
| |
| | |
Salbutamol, also known as albuterol and marketed as Ventolin among other names,[1] is a medication that opens up the medium and large airways in the lungs.[3] It is used to treat asthma, exercise-induced bronchospasm, and chronic obstructive pulmonary disease (COPD).[3] It may also be used to treat high blood potassium levels.[4] It is usually used by inhaler or nebulizer but is also available as a pill and intravenous solution.[3][5] Onset of action of the inhaled version is typically within 15 minutes and lasts for two to six hours.[3]
Common side effects include shakiness, headache, fast heart rate, dizziness, and feeling anxious. Serious side effects may include worsening bronchospasm, irregular heartbeat, and low blood potassium levels.[3] It can be used during pregnancy and breastfeeding, but safety is not entirely clear.[3][6] Salbutamol is a short-acting β2 adrenergic receptor agonist which works by causing airway smooth muscles to relax.[3]
Salbutamol was first made in 1967 in Britain.[7] It was approved for medical use in the United States in 1982.[3] It is on the World Health Organization's List of Essential Medicines, the most important medication needed in a basic health system.[8] It is available as a generic medication.[3][9] The wholesale cost in the developing world of an inhaler which contains 200 doses is between $1.12 and $2.64 (USD) as of 2014.[10] In the United States it is between $25 and $50 for a typical month supply.[11]
Medical uses
Salbutamol is typically used to treat bronchospasm (due to any cause, allergen asthma or exercise-induced), as well as chronic obstructive pulmonary disease.[12]
As a β2 agonist, salbutamol also finds use in obstetrics. Intravenous salbutamol can be used as a tocolytic to relax the uterine smooth muscle to delay premature labor. While preferred over agents such as atosiban and ritodrine, its role has largely been replaced by the calcium channel blocker nifedipine, which is more effective, better tolerated and orally administered.[13]
Salbutamol has been used to treat acute hyperkalemia, as it stimulates potassium flow into cells thus lowering the level in the blood.[4]
Adverse effects
The most common side effects are fine tremor, anxiety, headache, muscle cramps, dry mouth, and palpitation.[14] Other symptoms may include tachycardia, arrhythmia, flushing, myocardial ischemia (rare), and disturbances of sleep and behaviour.[14] Rarely occurring, but of importance, are allergic reactions of paradoxical bronchospasm, urticaria, angioedema, hypotension, and collapse. High doses or prolonged use may cause hypokalaemia, which is of concern especially in patients with renal failure and those on certain diuretics and xanthine derivatives.[14]
Chemistry
Structure and activity
-_and_(S)-salbutamol.svg.png)
This drug is sold as a racemic mixture. The (R)-(−)-enantiomer (CIP nomenclature) is shown in the image at right (top), and is responsible for the pharmacologic activity; the (S)-(+)-enantiomer (bottom) blocks metabolic pathways associated with elimination of itself and of the pharmacologically active enantiomer (R).[15]
With regard to structure-activity relationships, the tertiary butyl group in albuterol/salbutamol makes it more selective for β2 receptors.
Detection after dosing
Salbutamol may be quantified in blood or plasma; practical needs for this include to confirm a diagnosis of poisoning in hospitalized patients, or to aid in a forensic investigation. As well, urinary salbutamol concentrations are frequently measured in competitive sports programs, for which a level in excess of 1000 μg/L is considered to represent abuse.
The window of detection for urine testing is on the order of just 24 hours, given the relatively short elimination half-life of the drug,[16][17][18] estimated at between 5 and 6 hours following oral administration of 4 mg.[19]
Society and culture
Cost

The wholesale cost of an inhaler which contains 200 doses is between $1.12 and $2.64 (USD) in the developing world as of 2014.[10] In the United Kingdom the wholesale price of an inhaler which contains 200 doses is GB£1.50 as of 2015.[20] In the United States a typical month supply is between $25 and $50.[11]
In some countries compliance with the Montreal Protocol, which requires the banning of the use of ozone-layer depleting CFCs, has caused the price of inhalers to increase as much as ten-fold, as generics have been forced off the market from 2009 to 2013 by new patents obtained by pharmaceutical companies for non-CFC delivery systems.
Names
Salbutamol is the INN while albuterol is the USAN. The drug is usually manufactured and distributed as the sulfate salt (salbutamol sulfate).
It was first sold by Allen & Hanburys (UK) under the brand name Ventolin, and has been used for the treatment of asthma ever since.[21] The drug is marketed under many names worldwide.[1]
Doping
There is no compelling evidence that salbutamol and other β2 agonists can increase performance in healthy athletes.[22] In spite of this, salbutamol required "a declaration of Use in accordance with the International Standard for Therapeutic Use Exemptions" under the 2010 WADA prohibited list. This requirement was relaxed when the 2011 list was published to permit the use of "salbutamol (maximum 1600 micrograms over 24 hours) and salmeterol when taken by inhalation in accordance with the manufacturers’ recommended therapeutic regimen."[23][24]
According to two small and limited studies, performed on eight and 16 subjects, respectively, salbutamol increases performance on endurance exercise even for a person without asthma.[25][26][27]
Another study contradicts the above findings, however. The double blind, randomised test conducted on 12 non-asthmatic athletes concluded that salbutamol had a negligible effect on endurance performance. Nevertheless, the study also showed that the drug's bronchodilating effect may have improved respiratory adaptation at the beginning of exercise.[28]
Salbutamol has been shown to improve muscle weight in rats[29] and anecdotal reports hypothesise that it might be an alternative to clenbuterol for purposes of fat burning and muscle gain, with multiple studies supporting this claim.[30][31][32][33][34] Abuse of the drug may be confirmed by detection of its presence in plasma or urine, typically exceeding 1000 µg/L.[16]
History
Salbutamol was discovered in 1966 by a team led by David Jack at the Allen and Hanburys laboratory (a subsidiary of Glaxo) in Ware, Hertfordshire, England, and was launched as Ventolin in 1969.[35] In 2015, the US FDA approved a generic form under the name Proair Respiclick for Teva Pharmaceuticals.[9]
Research
Salbutamol has also been tested in a trial aimed at treatment of spinal muscular atrophy; it is speculated to modulate the alternative splicing of the SMN2 gene, increasing the amount of the SMN protein whose deficiency is regarded as a cause of the disease.[36][37]
It has been studied in subtypes of congenital myasthenic syndrome associated with mutations in Dok-7.[38]
See also
- Levosalbutamol — the (R)-(−)-enantiomer
- Ipratropium/salbutamol
- Salmeterol
- Isoprenaline
References
- 1 2 3 Drugs.com International brands of salbutamol Page accessed April 11, 2016
- ↑ Health Canada
- 1 2 3 4 5 6 7 8 9 10 11 "Albuterol". The American Society of Health-System Pharmacists. Retrieved Dec 2, 2015.
- 1 2 Mahoney, BA; Smith, WA; Lo, DS; Tsoi, K; Tonelli, M; Clase, CM (18 April 2005). "Emergency interventions for hyperkalaemia.". The Cochrane database of systematic reviews (2): CD003235. doi:10.1002/14651858.CD003235.pub2. PMID 15846652.
- ↑ Starkey, ES; Mulla, H; Sammons, HM; Pandya, HC (September 2014). "Intravenous salbutamol for childhood asthma: evidence-based medicine?". Archives of Disease in Childhood. 99 (9): 873–7. doi:10.1136/archdischild-2013-304467. PMID 24938536.
- ↑ Yaffe, Sumner J. (2011). Drugs in pregnancy and lactation : a reference guide to fetal and neonatal risk (9th ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 32. ISBN 9781608317080.
- ↑ Landau, Ralph (1999). Pharmaceutical innovation : revolutionizing human health. Philadelphia: Chemical Heritage Press. p. 226. ISBN 9780941901215.
- ↑ "WHO Model List of Essential Medicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- 1 2 "Approval History: NDA 205636". DRUGS@FDA. FDA.
- 1 2 3 "Salbutamol". International Drug Price Indicator Guide. Retrieved 5 December 2015.
- 1 2 Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 448. ISBN 9781284057560.
- ↑ "Albuterol". The American Society of Health-System Pharmacists. Retrieved 3 April 2011.
- ↑ Rossi, S (2004). Australian Medicines Handbook. AMH. ISBN 0-9578521-4-2.
- 1 2 3 "3.1.1.1 Selective beta2 agonists – side effects". British National Formulary (57 ed.). London: BMJ Publishing Group Ltd and Royal Pharmaceutical Society Publishing. March 2008. ISBN 0-85369-778-7.
- ↑ Mehta, Akul. [The tertiary butyl group in salbutamol (or albuterol) makes it more selective for β2 receptors. http://pharmaxchange.info/notes/medicinal_chemistry/adrenergics_cholinergics.html "Medicinal Chemistry of the Peripheral Nervous System – Adrenergics and Cholinergics their Biosynthesis, Metabolism, and Structure Activity Relationships"] Check
|url=value (help). Retrieved 2010-10-20. - 1 2 Baselt, R. (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Biomedical Publications. pp. 33–35. ISBN 0-9626523-6-9.
- ↑ Berges, Rosa; S; V; F; M; F; M; D (2000). "Discrimination of Prohibited Oral Use of Salbutamol from Authorized Inhaled Asthma Treatment". Clinical Chemistry. 46 (9): 1365–75. PMID 10973867.
- ↑ Schweizer, C; Saugy, M; Kamber, M (2004). "Doping test reveals high concentrations of salbutamol in a Swiss track and field athlete". Clin. J. Sport Med. 14 (5): 312–315. doi:10.1097/00042752-200409000-00018. PMID 15377972.
|first4=missing|last4=in Authors list (help) - ↑ "Albuterol Sulfate", Rx List: The Internet Drug Index, 6/12/2008, retrieved 2014-07-13 Check date values in:
|date=(help) - ↑ BNF 69: March 2015 - September 2015 (69 ed.). Pharmaceutical Pr. March 31, 2015. p. 190. ISBN 9780857111562.
- ↑ "Ventolin remains a breath of fresh air for asthma sufferers, after 40 years" (PDF). The Pharmaceutical Journal. 279 (7473): 404–405. Archived from the original (PDF) on Oct 15, 2007.
- ↑ Davis, E; Loiacono, R; Summers, R J (2008). "The rush to adrenaline: drugs in sport acting on the β-adrenergic system". British Journal of Pharmacology. 154 (3): 584–97. doi:10.1038/bjp.2008.164. PMC 2439523
 . PMID 18500380.
. PMID 18500380. - ↑ "THE 2010 PROHIBITED LIST INTERNATIONAL STANDARD" (PDF). WADA. Retrieved 2010-10-20.
- ↑ "THE 2011 PROHIBITED LIST INTERNATIONAL STANDARD" (PDF). WADA. Retrieved 2012-05-22.
- ↑ Collomp, K; Candau, R; Lasne, F; Labsy, Z; Préfaut, C; De Ceaurriz, J (2000). "Effects of short-term oral salbutamol administration on exercise endurance and metabolism". Journal of applied physiology (Bethesda, Md.: 1985). 89 (2): 430–6. PMID 10926623.
- ↑ "Salbutamol: Ergogenic effects of salbutamol". Retrieved 2010-10-20.
- ↑ Van Baak, MA; De Hon, OM; Hartgens, F; Kuipers, H (2004). "Inhaled salbutamol and endurance cycling performance in non-asthmatic athletes". International journal of sports medicine. 25 (7): 533–8. doi:10.1055/s-2004-815716. PMID 15459835.
- ↑ Goubault, C; Perault, MC; Leleu, E; Bouquet, S; Legros, P; Vandel, B; Denjean, A (2001). "Effects of inhaled salbutamol in exercising non-asthmatic athletes". Thorax. 56 (9): 675–679. doi:10.1136/thorax.56.9.675. PMC 1746141
 . PMID 11514686.
. PMID 11514686. - ↑ Carter WJ, Lynch ME (September 1994). "Comparison of the effects of salbutamol and clenbuterol on skeletal muscle mass and carcass composition in senescent rats". Metab. Clin. Exp. 43 (9): 1119–25. doi:10.1016/0026-0495(94)90054-X. PMID 7916118.
- ↑ Caruso, JF; Signorile, JF; Perry, AC; Leblanc, B; Williams, R; Clark, M; Bamman, MM (Nov 1995). "The effects of albuterol and isokinetic exercise on the quadriceps muscle group.". Medicine and science in sports and exercise. 27 (11): 1471–6. doi:10.1249/00005768-199511000-00002. PMID 8587482.
- ↑ Caruso, J. (20 January 2005). "Albuterol aids resistance exercise in reducing unloading-induced ankle extensor strength losses". Journal of Applied Physiology. 98 (5): 1705–1711. doi:10.1152/japplphysiol.01015.2004.
- ↑ Caruso, John F.; Hamill, John L.; De Garmo, Nicole (2005). "Oral Albuterol Dosing During the Latter Stages of a Resistance Exercise Program". The Journal of Strength and Conditioning Research. 19 (1): 102–7. doi:10.1519/R-14793.1. PMID 15705021.
- ↑ Caruso, JF; Hamill, JL; Yamauchi, M; Mercado, DR; Cook, TD; Keller, CP; Montgomery, AG; Elias, J (Jun 2004). "Albuterol helps resistance exercise attenuate unloading-induced knee extensor losses.". Aviation, space, and environmental medicine. 75 (6): 505–11. PMID 15198276.
- ↑ Caruso, JF; Hamill, JL; De Garmo, N (Feb 2005). "Oral albuterol dosing during the latter stages of a resistance exercise program.". Journal of strength and conditioning research / National Strength & Conditioning Association. 19 (1): 102–7. doi:10.1519/00124278-200502000-00018. PMID 15705021.
- ↑ "Sir David Jack, who has died aged 87, was the scientific brain behind the rise of the pharmaceuticals company Glaxo". Telegraph Newspaper. Nov 17, 2011.
- ↑ Van Meerbeke, J. P.; Sumner, C. J. (2011). "Progress and promise: The current status of spinal muscular atrophy therapeutics". Discovery medicine. 12 (65): 291–305. PMID 22031667.
- ↑ Lewelt, A.; Newcomb, T. M.; Swoboda, K. J. (2011). "New Therapeutic Approaches to Spinal Muscular Atrophy". Current Neurology and Neuroscience Reports. 12 (1): 42–53. doi:10.1007/s11910-011-0240-9. PMC 3260050
 . PMID 22134788.
. PMID 22134788. - ↑ Liewluck, Teerin; Selcen, Duygu; Engel, Andrew G. (November 2011). "Beneficial effects of albuterol in congenital endplate acetylcholinesterase deficiency and Dok-7 myasthenia". Muscle & Nerve. 44 (5): 789–794. doi:10.1002/mus.22176. PMC 3196786
 . PMID 21952943.
. PMID 21952943.
External links
- U.S. National Library of Medicine: Drug Information Portal – Albuterol
- Side Effects
- Salbutamol at The Periodic Table of Videos
