Solabegron
 | |
| Clinical data | |
|---|---|
| ATC code | None |
| Identifiers | |
| |
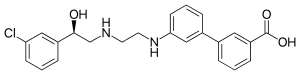
| Synonyms | 3-[3-[2-[[(2R)-2-(3-chlorophenyl)-2-hydroxyethyl]amino]ethylamino]phenyl]benzoic acid |
| CAS Number |
451470-34-1 |
| PubChem (CID) | 9887812 |
| ChemSpider |
8063484 |
| UNII |
55P6YH9O6N |
| KEGG |
D05879 |
| Chemical and physical data | |
| Formula | C23H23ClN2O3 |
| Molar mass | 410.892 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Solabegron (code name GW-427,353) is a drug which acts as a selective agonist for the β3 adrenergic receptor. It is being developed for the treatment of overactive bladder and irritable bowel syndrome.[1][2][3] It has been shown to produce visceral analgesia by releasing somatostatin from adipocytes.[4][5]
Solabegron was discovered by GlaxoSmithKline and acquired by AltheRx in March 2011. Solabegron relaxes the bladder smooth muscle by stimulating β3 adrenoceptors, a novel mechanism compared to older established drug treatments for overactive bladder syndrome such as the anticholinergic agents. Astellas Pharma has developed the first commercially available β3 adrenergic receptor, mirabegron, which is now licensed in Japan[6] and the U.S.[7] for overactive bladder. Mirabegron is not licensed for irritable bowel syndrome.
A Phase II study of solabegron for overactive bladder (OAB) looked at 258 patients with moderate-to-severe incontinence experiencing an average of 4.5 wet episodes per day. Results demonstrated a statistically significant improvement with solabegron as compared to placebo, as measured by the percent reduction of the number of wet episodes and the absolute number of daily voids.
A Phase II study for irritable bowel syndrome (IBS) evaluated 102 patients with IBS. Solabegron demonstrated significant reduction in pain associated with the disorder and a trend for greater improvement in the quality of life, compared to placebo.
Both Phase II studies indicated a tolerability profile for solabegron that was similar to placebo. The OAB patients did not suffer from dry mouth, constipation, increase in heart rate or cognitive issues.
AltheRx is currently preparing to advance solabegron into a large clinical study in OAB.
References
- ↑ Hicks, A; McCafferty, GP; Riedel, E; Aiyar, N; Pullen, M; Evans, C; Luce, TD; Coatney, RW; Rivera, GC; Westfall, TD; Hieble, JP (Oct 2007). "GW427353 (solabegron), a novel, selective beta3-adrenergic receptor agonist, evokes bladder relaxation and increases micturition reflex threshold in the dog". Journal of Pharmacology and Experimental Therapeutics. 323 (1): 202–9. doi:10.1124/jpet.107.125757. PMID 17626794.
- ↑ Grudell, AB; Camilleri, M; Jensen, KL; Foxx-Orenstein, AE; Burton, DD; Ryks, MD; Baxter, KL; Cox, DS; Dukes, GE; Kelleher, DL; Zinsmeister, AR (May 2008). "Dose-response effect of a beta3-adrenergic receptor agonist, solabegron, on gastrointestinal transit, bowel function, and somatostatin levels in health". American Journal of Physiology. Gastrointestinal and Liver Physiology. 294 (5): G1114–9. doi:10.1152/ajpgi.00051.2008. PMID 18372395.
- ↑ Kelleher, DL; Hicks, KJ; Cox, DS; et al. (2008). "Randomized, double-blind, placebo (PLA)-controlled, crossover study to evaluate efficacy and safety of the beta 3-adrenergic receptor agonist solabegron (SOL) in patients with irritable bowel syndrome (IBS)". Neurogastroenterol Motil. 20 (Suppl 2): 131.
- ↑ Cellek, S; Thangiah, R; Bassil, AK; Campbell, CA; Gray, KM; Stretton, JL; Lalude, O; Vivekanandan, S; Wheeldon, A; Winchester, WJ; Sanger, GJ; Schemann, M; Lee, K (Jul 2007). "Demonstration of functional neuronal beta3-adrenoceptors within the enteric nervous system". Gastroenterology. 133 (1): 175–83. doi:10.1053/j.gastro.2007.05.009.
- ↑ Schemann, M; Hafsi, N; Michel, K; Kober, OI; Wollmann, J; Li, Q; Zeller, F; Langer, R; Lee, K; Cellek, S (2009). "The beta3-adrenoceptor agonist GW427353 (solabegron) decreases excitability of human enteric neurons via release of somatostatin". Gastroenterology.
- ↑ "Mirabegron for the treatment of overactive bladder". Drugs of Today. 48 (1): 25–32. January 2012. doi:10.1358/dot.2012.48.1.1738056. PMID 22384458.
- ↑ http://chembl.blogspot.co.uk/2012/07/new-drug-approvals-2012-pt-xiv.html