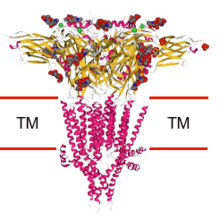
5-HT3 receptor
The 5-HT3 receptor belongs to the Cys-loop superfamily of ligand-gated ion channels (LGICs) and therefore differs structurally and functionally from all other 5-HT receptors (5-hydroxytryptamine, or serotonin) receptors which are G protein-coupled receptors.[1][2][3] This ion channel is cation-selective and mediates neuronal depolarization and excitation within the central and peripheral nervous systems.[1] As with other ligand gated ion channels, the 5-HT3 receptor consists of five subunits arranged around a central ion conducting pore, which is permeable to sodium (Na), potassium (K), and calcium (Ca) ions. Binding of the neurotransmitter 5-hydroxytryptamine (serotonin) to the 5-HT3 receptor opens the channel, which, in turn, leads to an excitatory response in neurons. The rapidly activating, desensitizing, inward current is predominantly carried by sodium and potassium ions.[2] 5-HT3 receptors have a negligible permeability to anions.[1] They are most closely related by homology to the nicotinic acetylcholine receptor.
Structure
The 5-HT3 receptor differs markedly in structure and mechanism from the other 5-HT receptor subtypes, which are all G-protein-coupled. A functional channel may be composed of five identical 5-HT3A subunits (homopentameric) or a mixture of 5-HT3A and one of the other four 5-HT3B,[7][8][9][10] 5-HT3C, 5-HT3D, or 5-HT3E subunits (heteropentameric).[11] It appears that only the 5-HT3A subunits form functional homopentameric channels. All other subunit subtypes must heteropentamerize with 5-HT3A subunits to form functional channels. Additionally, there has not currently been any pharmacological difference found between the heteromeric 5-HT3AC, 5-HT3AD, 5-HT3AE, and the homomeric 5-HT3A receptor.[12]
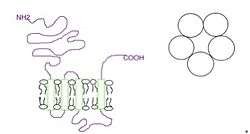
The subunits surround a central ion channel in a pseudo-symmetric manner (Fig.1). Each subunit comprises an extracellular N-terminal domain which comprises the orthosteric ligand-binding site; a transmembrane domain consisting of four interconnected alpha helices (M1-M4), with the extracellular M2-M3 loop involved in the gating mechanism; a large cytoplasmic domain between M3 and M4 involved in receptor trafficking and regulation; and a short extracellular C-terminus (Fig.1).[1] Whereas extracellular domain is the site of action of agonists and competitive antagonists, the transmembrane domain contains the central ion pore, receptor gate, and principle selectivity filter that allows ions to cross the cell membrane.[2]
Gene
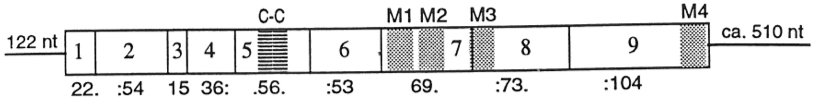
The 5-HT3 receptor gene is located on human chromosomal region 11q23.1-q23.2. It is similar in structure to the mouse gene which has 9 exons and is spread over ~13 kb. Interestingly, four of its introns are exactly in the same position as the introns in the homologous α7-Acetylcholine receptor gene, clearly proving their evolutionary relationship.[13][14] Genes that code for the subunits of the 5-HT3 receptor have been identified. HTR3A and HTR3B for the 5-HT3A and 5-HT3B subunits and in addition HTR3C, HTR3D and HTR3E genes encoding 5-HT3C, 5-HT3D and 5-HT3E subunits. The latter three tend to show peripherally restricted pattern of expression, with high levels in the gut. In human duodenum and stomach, for example, 5-HT3C and 5-HT3E mRNA might be greater than for 5-HT3A and 5-HT3B. There is some evidence to suggest that the 5-HT3 receptor subunits are an important contribution to the effectiveness of these compounds.[2] In patients treated with chemotherapeutic drugs, certain polymorphism of the HTR3B gene could predict successful antiemetic treatment. This could indicate that the 5-HT3B receptor subunit could be used as biomarker of antiemetic drug efficacy. HTR3C and HTR3E do not seem to form functional homomeric channels, but when co-expressed with HTR3A they form heteromeric complex with decreased or increased 5-HT efficacies. The pathophysiological role for these additional subunits has yet to be identified.[15]
Tissue distribution
The 5-HT3 receptor is expressed throughout the central and peripheral nervous systems and mediates a variety of physiological functions.[16] On a cellular level, it has been shown that postsynaptic 5-HT3 receptors mediate fast excitatory synaptic transmission in rat neocortical interneurons, amygdala, and hippocampus, and in ferret visual cortex.[17][18][19][20] 5-HT3 receptors are also present on presynaptic nerve terminals. There is some evidence for a role in modulation of neurotransmitter release,[21][22] but evidence is inconclusive.[23]
Effects
When the receptor is activated to open the ion channel by agonists, the following effects are observed:
- CNS: nausea and vomiting center in brain stem, anxiety,[24] seizure propensity [25]
- PNS: neuronal excitation (in autonomic, nociceptive neurons), emesis[24]
Agonists
Agonists for the receptor include:
- 2-methyl-5-HT
- Alpha-Methyltryptamine
- Bufotenin
- Chlorophenylbiguanide[24]
- Ethanol
- Ibogaine
- Phenylbiguanide
- Quipazine
- RS-56812: Potent and selective 5-HT3 partial agonist, 1000x selectivity over other serotonin receptors
- SR-57227
- Varenicline[26]
- YM-31636[27]
Antagonists
Antagonists for the receptor (sorted by their respective therapeutic application) include:
- Antiemetics
- Gastroprokinetics
- Alosetron
- Batanopride
- Metoclopramide (high doses)
- Renzapride
- Zacopride
- M1, the major active metabolite of mosapride
- Antidepressants
- Antipsychotics
- Others
- 3-Tropanyl indole-3-carboxylate
- Lamotrigine (Epilepsy and Bipolar Disorder)
- Memantine (Alzheimer's disease medication)
- Menthol [28]
- Thujone
Positive Allosteric Modulators
These agents are not agonists at the receptor, but increase the affinity or efficacy of the receptors for an agonist:
- 5-chloroindole [29]
Discovery
Identification of the 5-HT3 receptor did not take place until 1986 because of a lack of selective pharmacological tool.[16] However, with the discovery that the 5-HT3 receptor plays a prominent role in chemotherapy- and radiotherapy-induced vomiting, and the concomitant development of selective 5-HT3 receptor antagonists to suppress these side effects aroused intense interest from the pharmaceutical industry[2][30] and therefore the identification of 5-HT3 receptors in cell lines and native tissues quickly followed.[16]
See also
References
- 1 2 3 4 Barnes NM, Hales TG, Lummis SC, Peters JA (January 2009). "The 5-HT3 receptor--the relationship between structure and function". Neuropharmacology. 56 (1): 273–84. doi:10.1016/j.neuropharm.2008.08.003. PMID 18761359.
- 1 2 3 4 5 Thompson AJ, Lummis SC (2006). "5-HT3 Receptors". Current Pharmaceutical Design. 12 (28): 3615–30. doi:10.2174/138161206778522029. PMC 2664614
 . PMID 17073663.
. PMID 17073663. - ↑ Reeves DC, Lummis SC (2002). "The molecular basis of the structure and function of the 5-HT3 receptor: a model ligand-gated ion channel (review)". Molecular Membrane Biology. 19 (1): 11–26. doi:10.1080/09687680110110048. PMID 11989819.
- ↑ Hassaine G, Deluz C, Grasso L, Wyss R, Tol MB, Hovius R, Graff A, Stahlberg H, Tomizaki T, Desmyter A, Moreau C, Li XD, Poitevin F, Vogel H, Nury H (2014). "X-ray structure of the mouse serotonin 5-HT3 receptor". Nature. 512 (7514): 276–81. doi:10.1038/nature13552. PMID 25119048.
- ↑ X-ray structure of the mouse serotonin 5-HT3 receptor in PDB
- ↑ Kudryashev M, Castaño-Díez D, Deluz C, Hassaine G, Grasso L, Graf-Meyer A, Vogel H, Stahlberg H (2015). "The Structure of the Mouse Serotonin 5-HT3 Receptor in Lipid Vesicles". Structure. doi:10.1016/j.str.2015.11.004. PMID 26724993.
- ↑ Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, Kirkness EF (1999). "The 5-HT3B subunit is a major determinant of serotonin-receptor function". Nature. 397 (6717): 359–63. doi:10.1038/16941. PMID 9950429.
- ↑ Dubin AE, Huvar R, D'Andrea MR, Pyati J, Zhu JY, Joy KC, Wilson SJ, Galindo JE, Glass CA, Luo L, Jackson MR, Lovenberg TW, Erlander MG (1999). "The pharmacological and functional characteristics of the serotonin 5-HT3A receptor are specifically modified by a 5-HT3B receptor subunit". J Biol Chem. 274 (43): 30799–810. doi:10.1074/jbc.274.43.30799. PMID 10521471.
- ↑ Monk SA, Desai K, Brady CA, Williams JM, Lin L, Princivalle A, Hope AG, Barnes NM (2001). "Generation of a selective 5-HT3B subunit-recognising polyclonal antibody; identification of immunoreactive cells in rat hippocampus". Neuropharmacology. 41 (8): 1013–6. doi:10.1016/S0028-3908(01)00153-8. PMID 11747906.
- ↑ Boyd GW, Low P, Dunlop JI, Ward M, Vardy AW, Lambert JJ, Peters J, Conolly CN (2002). "Assembly and cell surface expression of homomeric and heteromeric 5-HT3 receptors: The role of oligomerisation and chaperone proteins". Mol Cell Neurosci. 21 (1): 38–50. doi:10.1006/mcne.2002.1160. PMID 12359150.
- ↑ Niesler B, Walstab J, Combrink S, Moeller D, Kapeller J, Rietdorf J, Boenisch H, Goethert M, Rappold G, Bruess M (2007). "Characterization of the Novel Human Serotonin Receptor Subunits 5-HT3C, 5- HT3D and 5-HT3E". Mol Pharmacol. 71 (Mar 28): 8–17. doi:10.1124/mol.106.032144. PMID 17392525.
- ↑ Niesler, Beate (February 2011). "5-HT3 receptors: potential of individual isoforms for personalised therapy". Current Opinion in Pharmacology. 11 (1): 81–86. doi:10.1016/j.coph.2011.01.011. PMID 21345729.
- 1 2 Uetz, P; Abdelatty, F; Villarroel, A; Rappold, G; Weiss, B; Koenen, M (1994). "Organisation of the murine 5-HT3 receptor gene and assignment to human chromosome 11". FEBS Letters. 339 (3): 302–6. doi:10.1016/0014-5793(94)80435-4. PMID 8112471.
- ↑ Uetz, P. (1992) Das 5HT3-Rezeptorgen der Maus. Diploma Thesis, University of Heidelberg, 143 pp.
- ↑ Sanger GJ (September 2008). "5-hydroxytryptamine and the gastrointestinal tract: where next?". Trends in Pharmacological Sciences. 29 (9): 465–71. doi:10.1016/j.tips.2008.06.008. PMID 19086255.
- 1 2 3 Yakel, JL (2000). Endo, M; Kurachi, Y; Mishina, M, eds. The 5-HT3 receptor channel: function, activation and regulation in Pharmacology of Ionic Channel Function: Activators and Inhibitors (Handbook of Experimental Pharmacology). 147. Berlin: Springer-Verlag. pp. 541–560. ISBN 3-540-66127-1.
- ↑ Férézou I, Cauli B, Hill EL, Rossier J, Hamel E, Lambolez B (2002). "5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons". J Neurosci. 22 (17): 7389–97. PMID 12196560.
- ↑ Kazuyoshi Kawa (1994). "Distribution and Functional Properties of 5HT3 Receptors in the Rat Hippocampus Dentate Gyrus". Journal of Neurophysiology. 71 (5): 1935–47. PMID 7520482.
- ↑ Sugita S, Shen KZ, North RA (1992). "5-hydroxytryptamine is a fast excitatory transmitter at 5-HT3 receptors in rat amygdala". Neuron. 8 (1): 199–203. doi:10.1016/0896-6273(92)90121-S. PMID 1346089.
- ↑ Roerig B, Nelson DA, Katz LC (1992). "Fast synaptic signaling by nicotinic acetylcholine and serotonin 5-HT3 receptors in developing visual cortex". J Neurosci. 17 (21): 199–203. PMID 9334409.
- ↑ Rondé P, Nichols RA (1998). "High calcium permeability of serotonin 5-HT3 receptors on presynaptic nerve terminals from rat striatum". J Neurochem. 70 (3): 1094–103. doi:10.1046/j.1471-4159.1998.70031094.x. PMID 9489730.
- ↑ Rondé P, Nichols RA (1997). "5-HT3 receptors induce rises in cytosolic and nuclear calcium in NG108-15 cells via calcium-induced calcium release". Cell Calcium. 22 (5): 357–65. doi:10.1016/S0143-4160(97)90020-8. PMID 9448942.
- ↑ van Hooft JA, Vijverberg HP (2000). "5-HT3 receptors and neurotransmitter release in the CNS: a nerve ending story?". Trends Neurosci. 23 (12): 605–10. doi:10.1016/S0166-2236(00)01662-3. PMID 11137150.
- 1 2 3 4 5 Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. ISBN 0-443-07145-4. Page 187
- ↑ Gholipour T, Ghasemi M, Riazi K, Ghaffarpour M, Dehpour AR (January 2010). "Seizure susceptibility alteration through 5-HT(3) receptor: modulation by nitric oxide". Seizure. 19 (1): 17–22. doi:10.1016/j.seizure.2009.10.006. PMID 19942458.
- ↑ Mineur YS, Picciotto MR (December 2010). "Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis". Trends Pharmacol. Sci. 31 (12): 580–6. doi:10.1016/j.tips.2010.09.004. PMC 2991594
 . PMID 20965579.
. PMID 20965579. - ↑ Imanishi, N.; Iwaoka, K.; Koshio, H.; Nagashima, S. Y.; Kazuta, K. I.; Ohta, M.; Sakamoto, S.; Ito, H.; Akuzawa, S.; Kiso, T.; Tsukamoto, S. I.; Mase, T. (2003). "New thiazole derivatives as potent and selective 5-hydroxytriptamine 3 (5-HT3) receptor agonists for the treatment of constipation". Bioorganic & Medicinal Chemistry. 11 (7): 1493. doi:10.1016/S0968-0896(02)00557-6.
- ↑ Ashoor, A.; Nordman, J.; Veltri, D.; Susan Yang, K. -H.; Shuba, Y.; Al Kury, L.; Sadek, B.; Howarth, F. C.; Shehu, A.; Kabbani, N.; Oz, M. (2013). "Menthol Inhibits 5-Ht3 Receptor-Mediated Currents". Journal of Pharmacology and Experimental Therapeutics. 347 (2): 398–409. doi:10.1124/jpet.113.203976. PMID 23965380.
- ↑ Newman, A. S.; Batis, N; Grafton, G; Caputo, F; Brady, C. A.; Lambert, J. J.; Peters, J. A.; Gordon, J; Brain, K. L.; Powell, A. D.; Barnes, N. M. (2013). "5-Chloroindole: A potent allosteric modulator of the 5-HT3 receptor". British Journal of Pharmacology. 169 (6): 1228–38. doi:10.1111/bph.12213. PMC 3831704
 . PMID 23594147.
. PMID 23594147. - ↑ Thompson AJ, Lummis SC (2007). "The 5-HT3 receptor as a therapeutic target". Expert Opin Ther Targets. 11 (4): 527–40. doi:10.1517/14728222.11.4.527. PMC 1994432
 . PMID 17373882.
. PMID 17373882.
External links
- 5-HT3 Receptor at the US National Library of Medicine Medical Subject Headings (MeSH)