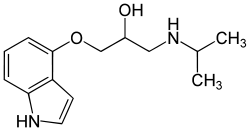
Pindolol
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Visken (originator), many generic brands[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684032 |
| Pregnancy category | |
| Routes of administration | oral, iv |
| ATC code | C07AA03 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 50% to 95% |
| Metabolism | Hepatic |
| Biological half-life | 3–4 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
13523-86-9 |
| PubChem (CID) | 4828 |
| IUPHAR/BPS | 91 |
| DrugBank |
DB00960 |
| ChemSpider |
4662 |
| UNII |
BJ4HF6IU1D |
| KEGG |
D00513 |
| ChEBI |
CHEBI:8214 |
| ChEMBL |
CHEMBL500 |
| ECHA InfoCard | 100.033.501 |
| Chemical and physical data | |
| Formula | C14H20N2O2 |
| Molar mass | 248.321 g/mol |
| 3D model (Jmol) | Interactive image |
| Chirality | Racemic mixture |
| |
| |
| | |
Pindolol (originally marketed as Visken, now marketed under many brands as a generic drug[1]) is a beta blocker.
Medical use
Pindolol is used for hypertension in the US, Canada, and Europe, and also for angina pectoris outside the US.[2] When used alone for hypertension, pindolol can significantly lower blood pressure and heart rate, but the evidence base for its use is weak, as the number of subjects in published studies is small.[2]
In some countries pindolol is also used for arrhythmias and prophylaxis of acute stress reactions.
Contraindications
Similar to propranolol with an extra contraindication for hyperthyroidism. In patients with thyrotoxicosis, possible deleterious effects from long-term use of pindolol have not been adequately appraised. Beta-blockade may mask the clinical signs of continuing hyperthyroidism or complications, and give a false impression of improvement. Therefore, abrupt withdrawal of pindolol may be followed by an exacerbation of the symptoms of hyperthyroidism, including thyroid storm.[3]
Pindolol has modest beta-adrenergic agonist activity and is therefore used with caution in angina pectoris.
Pharmacology
Pindolol is a nonselective beta blocker with partial beta-adrenergic receptor agonist activity and also possesses intrinsic sympathomimetic activity. This means that pindolol, particularly in high doses, exerts effects like epinephrine or isoprenaline (increased pulse rate, increased blood pressure, bronchodilation), but these effects are limited. Pindolol also shows membrane stabilizing effects like quinidine, possibly accounting for its antiarrhythmic effects. It also functions as a 5-HT1A receptor weak partial agonist / antagonist (Ki=33nM[4]).
Pharmacokinetics
Pindolol is rapidly and well absorbed from the GI tract. It undergoes some first-pass-metabolization leading to an oral bioavailability of 50 to 95%. Patients with uremia may have a reduced bioavailability. Food does not alter the bioavailability, but may increase the resorption. Following an oral single dose of 20 mg peak plasma concentrations are reached within 1 to 2 hours. The effect of pindolol on pulse rate (lowering) is evident after 3 hours. Despite the rather short halflife of 3 to 4 hours, hemodynamic effects persist for 24 hours after administration. Plasma halflives are increased to 3 - 11.5 hours in patients with renal impairment, to 7 – 15 hours in elderly patients, and from 2.5 to 30 hours in patients with liver cirrhosis. Approximately 2/3 of pindolol are metabolized in the liver giving hydroxylates, which are found in the urine as gluconurides and ethereal sulfates. The remaining 1/3 of pindolol is excreted in urine in unchanged form.
History
Pindolol was patented by Sandoz in 1966 and was launched in the US in 1977.[5]
Research
- Augmentation therapy of clinical depression: Pindolol is sometimes added to a standard antidepressant therapy,[6] if the patient fails to respond to the standard therapy alone. Fluoxetine is the most commonly used standard antidepressant. The results of augmentation therapy are encouraging. It is not known whether pindolol has antidepressive activities as monotherapeutic agent.
- Pindolol is a potent scavenger of nitric oxide. This affect is potentiated by sodium bicarbonate. Inhibition of nitric oxide synthesis has an anxiolytic effect in animals.[7]
- Augmentation therapy of premature ejaculation: According to a recent study, pindolol can be effectively added to a standard anti-premature-ejaculation therapy, which usually consists of daily doses of an SSRI antidepressant such as fluoxetine or paroxetine. Augmentation of pindolol results in substantial increase of ejaculatory latency, even in those who previously did not experience in an improvement with the SSRI monotherapy.[8]
References
- 1 2 Drugs.com International brand names for pindolol Page accessed Sept 4, 2015
- 1 2 Wong, GW; Boyda, HN; Wright, JM (Nov 2014). "Blood pressure lowering efficacy of partial agonist beta blocker monotherapy for primary hypertension". Cochrane Database Syst Rev. 11: CD007450. doi:10.1002/14651858.CD007450.pub2. PMID 25427719.
- ↑ http://www.rxmed.com/b.main/b2.pharmaceutical/b2.1.monographs/CPS-%20Monographs/CPS-%20%28General%20Monographs-%20V%29/VISKEN.html
- ↑ "PDSP". Retrieved 12 June 2013.
- ↑ "Discovery and Development of Major Drugs. Chapter 2 in Pharmaceutical Innovation: Revolutionizing Human Health. Volume 2 of Chemical Heritage Foundation series in innovation and entrepreneurship. Eds Ralph Landau, Basil Achilladelis, Alexander Scriabine. Chemical Heritage Foundation, 1999. ISBN 9780941901215 p 185
- ↑ Isaac MT (November 2004). "Review: combining pindolol with an SSRI improves early outcomes in people with depression". Evid Based Ment Health. 7 (4): 107. doi:10.1136/ebmh.7.4.107. PMID 15504795.
- ↑ "Pindolol is a potent scavenger of reactive nitrogen species". Life Sciences. 77: 1983–1992. doi:10.1016/j.lfs.2005.02.018.
- ↑ Safarinejad, MR (2008). "Once-daily high-dose pindolol for paroxetine-refractory premature ejaculation: a double-blind, placebo-controlled and randomized study.". Journal of Clinical Psychopharmacology. 28 (1): 39–44. doi:10.1097/jcp.0b013e31816073a5. PMID 18204339.