Mazindol
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | Oral |
| ATC code | A08AA05 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 93% |
| Metabolism | Hepatic |
| Biological half-life | 10-13 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
22232-71-9 |
| PubChem (CID) | 4020 |
| IUPHAR/BPS | 4797 |
| DrugBank |
DB00579 |
| ChemSpider |
3880 |
| UNII |
C56709M5NH |
| KEGG |
D00367 |
| ChEMBL |
CHEMBL781 |
| ECHA InfoCard | 100.040.764 |
| Chemical and physical data | |
| Formula | C16H13ClN2O |
| Molar mass | 284.74 g/mol |
| 3D model (Jmol) | Interactive image |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Mazindol (Mazanor, Sanorex) is a stimulant drug of the tetracyclic chemical class which is used as an anorectic.[1] It was developed by Sandoz-Wander in the 1960s.[2]
Indications
Mazindol is used in short-term (i.e., a few weeks) treatment of exogenous obesity, in combination with a regimen of weight reduction based on caloric restriction, exercise, and behavior modification in patients with a body mass index of 30 kg of body weight per height in meters squared (kg/m²), or in patients with a body mass index of 27 kg/m2 in the presence of risk factors such as hypertension, diabetes, or kghyperlipidemia. Mazindol is not currently available as a commercially marketed and FDA regulated prescription agent for the treatment of obesity.
Pharmacology
Mazindol is a sympathomimetic amine, which is similar to amphetamine. It stimulates the central nervous system, which increases heart rate and blood pressure, and decreases appetite. Sympathomimetic anoretics (appetite suppressants) are used in the short-term treatment of obesity. Their appetite-reducing effect tends to decrease after a few weeks of treatment. Because of this, these medicines are useful only during the first few weeks of a weight-loss program.
Although the mechanism of action of the sympathomimetics in the treatment of obesity is not fully known, these medications have pharmacological effects similar to those of amphetamines. Like other sympathomimetic appetite suppressants, mazindol is thought to act as a reuptake inhibitor of norepinephrine. In addition, it inhibits dopamine and serotonin reuptake. The recommended dosage is 2 mg per day for 90 days in patients 40 kg overweight and under; 4 mg a day in patients more than 50 kg overweight; separated into two doses separated by a 12-hour window between each 2 mg dose.
Overdose
Symptoms of a mazindol overdose include: restlessness, tremor, rapid breathing, confusion, hallucinations, panic, aggressiveness, nausea, vomiting, diarrhea, an irregular heartbeat, and seizures.
Analogs
From considering the attached QSAR table we can make the apparent observations:[3]
- Desoxylation of the tertiary alcohol in mazindol improves DAT and SERT binding. The compound now behaves as a functional SNDRI instead of predominantly a noradrenaline reuptake inhibitor.
- Removal of the p-chloro atom in mazindol means that the compound now only behaves as a noradrenaline reuptake inhibitor.
- Changing the size of the imidazoline type ring system in mazindol to the corresponding six-membered homolog increases potency of the resultant compound at the DAT by approximately ten-fold.
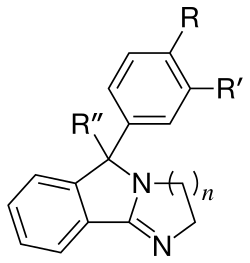
| n | R | R' | R" | hSERT | hNET | hDAT |
|---|---|---|---|---|---|---|
| 1 | Cl | H | OH | 94 | 4.9 | 43 |
| 1 | Cl | H | H | 15 | 6.9 | 6.0 |
| 1 | H | H | OH | 2140 | 2.8 | 730 |
| 1 | -C4H4- | OH | 1.8 | 4.5 | 66 | |
| 2 | Cl | H | OH | 53 | 4.9 | 3.7 |
| 2 | OH | H | OH | 60 | 1.9 | 59.0 |
| 2 | OMe | H | OH | 94 | 4.1 | 30.4 |
| 2 | -OCH2O- | OH | 83 | 0.62 | 2.21 | |
To make the six membered (so-called homomazindol) analog, one can simply substitute 1,2-diaminoethane with 1,3-diaminopropane. Importantly, another procedure was also published.
Given the data in the above table, one might also be interested in making the desoxy-mazindol analog. The synthesis for this is facile. Although "mazindane" is relatively stable in air, it is readily oxidized to mazindol upon contact with monoamine oxidaze enzymes present at the DAT.
| Compound | S. Singh's alphanumeric assignation (name) |
R | R′ | R′′ | IC50 (nM) (Inhibition of [3H]WIN 35428 binding) |
IC50 (nM) (Inhibition of [3H]DA uptake) |
Selectivity uptake/binding |
|---|---|---|---|---|---|---|---|
| (cocaine) | 89.1 ± 8 | 208 ± 12 | 2.3 | ||||
| (mazindol) | H | H | 4′-Cl | 8.1 ± 1.2 | 8.4 ± 1.3 | 1.0 | |
| 384a | H | H | H | 66.0 ± 8.9 | 124 ± 37 | 1.9 | |
| 384b | H | H | 4′-F | 13.3 ± 1.8 | 25.4 ± 2.7 | 1.9 | |
| 384c | H | 7-F | H | 29.7 ± 7.0 | 78 ± 46 | 2.6 | |
| 384d | H | H | 2′-Cl | 294 ± 6 | 770 ± 159 | 2.6 | |
| 384e | H | H | 3′-Cl | 4.3 ± 0.4 | 9.2 ± 5.3 | 2.1 | |
| 384f | CH3 | H | 4′-Cl | 50.4 ± 5.5 | 106 ± 5.6 | 2.1 | |
| 384g | H | 6-Cl | H | 57.2 ± 8.3 | 58 ± 6.4 | 1.0 | |
| 384h | H | 7-Cl | H | 85.4 ± 14 | 55.17 | 0.6 | |
| 384i | H | 7-F | 4′-Cl | 6.5 ± 1.2 | 15 ± 9 | 2.3 | |
| 384j | H | 7-Cl | 4′-F | 52.8 ± 8.7 | 53 ± 18 | 1.0 | |
| 384k | H | H | 2′,4′-Cl2 | 76.5 ± 1.11 | 92 ± 19 | 1.2 | |
| 384l | H | H | 3′,4′-Cl2 | 2.5 ± 0.5 | 1.4 ± 1.6 | 0.6 | |
| 384m | H | 7,8-Cl2 | 4′-Cl | 13.6 ± 1.5 | |||
| 384n | H | H | 2′-Br | 1340 ± 179 | |||
| 384o | H | H | 4′-Br | 2.6 ± 1.5 | 8.6 ± 3.5 | 3.3 | |
| 384p | H | H | 4′-I | 17.2 ± 0.9 | 14 ± 6.4 | 0.8 |
| Compound | S. Singh's alphanumeric assignation (name) |
R | R′ | IC50 (nM) (Inhibition of [3H]WIN 35428 binding) |
IC50 (nM) (Inhibition of [3H]DA uptake) |
Selectivity uptake/binding |
|---|---|---|---|---|---|---|
| 388a | H | H | 5.8 ± 1.6 | 18 ± 11 | 3.1 | |
| 388b | H | 2′-F | 23.2 ± 1.7 | 89 ± 2.8 | 3.8 | |
| 388c | H | 3′-F | 2.0 ± 0.02 | 3.1 ± 1.8 | 1.6 | |
| 388d | H | 4′-F | 3.2 ± 1.7 | 8.5 ± 4.9 | 0.4 | |
| 388e | H | 3′-Cl | 1.0 ± 0.2 | 1.3 ± 0.14 | 1.3 | |
| 388f | H | 4′-Cl | 1.7 ± 0.2 | 1.4 ± 0.35 | 0.8 | |
| 388g | CH3 | 4′-Cl | 6.3 ± 4.5 | 1.7 ± 1.6 | 0.3 | |
| 389a | H | 5.9 ± 0.1 | 11 ± 3.2 | 2.0 | ||
| 389b | 4′-Cl | 1.5 ± 0.1 | 3.4 ± 2.3 | 2.3 | ||
| 389c | 3′,4′-Cl2 | 1.7 ± 0.1 | 0.26 ± 0.16 | 0.2 |
See also: SNDRI for context.
Synthesis
Preparation starts by reaction of a substituted benzoylbenzoic acid (1) with ethylenediamine The product 3 can be rationalized as being an aminal from the initially formed monoamide 2. This is then subjected to reduction with LiAlH4 and-without isolation-air oxidation. Reduction probably proceeds to the mixed aminal/carbinolamine 4; such a product would be expected to be in equilibrium with the alternate aminal 5. The latter would be expected to predominate because of the greater stability of aldehyde aminals over the corresponding ketone derivatives. Air oxidation of the tetrahydroimidazole to the imidazoline will then remove 5 from the equilibrium. There is thus obtained the anorectic agent mazindol (6).
See also
References
- ↑ Carruba, Michele O.; Zambotti, Fernanda; Vicentini, Lucia; Picotti, Giovanni B.; Mantegazza, Paolo (1978). "Pharmacology and biochemical profile of a new anorectic drug: mazindol". Cent. Mech. Anorectic Drugs: 145–64.
- ↑ US Patent 3597445 - 1H-Isoindole Intermediates
- ↑ Houlihan, W. J.; Ahmad, U. F.; Koletar, J.; Kelly, L.; Brand, L.; Kopajtic, T. A. (2002). "Benzo- and cyclohexanomazindol analogues as potential inhibitors of the cocaine binding site at the dopamine transporter". Journal of Medicinal Chemistry. 45 (19): 4110–4118. doi:10.1021/jm010301z. PMID 12213054.Houlihan, W. J.; Kelly, L.; Pankuch, J.; Koletar, J.; Brand, L.; Janowsky, A.; Kopajtic, T. A. (2002). "Mazindol analogues as potential inhibitors of the cocaine binding site at the dopamine transporter". Journal of Medicinal Chemistry. 45 (19): 4097–4109. doi:10.1021/jm010302r. PMID 12213053.
- 1 2 Chemistry, Design, and Structure-Activity Relationship of Cocaine Antagonists. Satendra Singh et al. Chem. Rev. 2000, 100. 925-1024. PubMed; Chemical Reviews (Impact Factor: 45.66). 04/2000; 100(3):925-1024 American Chemical Society; 2000) ChemInform; May, 16th 2000, Volume 31, Issue 20., ISSN 0009-2665, doi:10.1002/chin.200020238. Mirror hotlink.
- ↑ Aeberli, Paul; Eden, Phillip; Gogerty, John H.; Houlihan, William J.; Penberthy, Chris (1975). "5-Aryl-2,3-dihydro-5H-imidazo[2,1-a]isoindol-5-ols. Novel class of anorectic agents". Journal of Medicinal Chemistry. 18 (2): 177. doi:10.1021/jm00236a014. PMID 804553.
- ↑ W. J. Houlihan, DE 1814540 C.A. 71, 81368s (1969).
- ↑ W. J. Houlihan, M. K. Eberle, DE 1930488; eidem, U.S. Patent 3,597,445 (1969, 1970, 1970 all to Sandoz).
- ↑ T. S. Sulkowski, U.S. Patent 3,763,178 (1973 to American Home Products).
