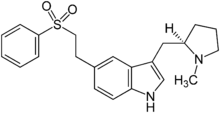
Eletriptan
 | |
| Clinical data | |
|---|---|
| Trade names | Relpax |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603029 |
| Pregnancy category |
|
| Routes of administration | oral |
| ATC code | N02CC06 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 50% |
| Metabolism | CYP3A4 |
| Biological half-life | 4 hours |
| Identifiers | |
| |
| CAS Number |
143322-58-1 |
| PubChem (CID) | 77993 |
| IUPHAR/BPS | 40 |
| DrugBank |
DB00216 |
| ChemSpider |
70379 |
| UNII |
22QOO9B8KI |
| KEGG |
D01973 |
| ChEBI |
CHEBI:50922 |
| ChEMBL |
CHEMBL1510 |
| ECHA InfoCard | 100.167.337 |
| Chemical and physical data | |
| Formula | C22H26N2O2S |
| Molar mass | 382.52 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Eletriptan (trade name Relpax, used in the form of eletriptan hydrobromide) is a second generation triptan drug intended for treatment of migraine headaches. It is used as an abortive medication, blocking a migraine attack which is already in progress. Eletriptan is marketed and manufactured by Pfizer Inc. It is sold in the US and Canada under the brand name Relpax, and in several other countries under the brand name Relert.
Approval and availability
Eletriptan was approved by the U.S. Food and Drug Administration (FDA) on December 26, 2002, for the acute treatment of migraine with or without aura in adults.[1] It is available only by prescription in the United States and Canada. It is not intended for the prophylactic therapy of migraine or for use in the management of hemiplegic or basilar migraine. It is available in 20 mg, 40 mg and 80 mg strengths.
Eletriptan is covered by U.S. Patent no. 5545644[1][2] and U.S. Patent no. 6110940;[1][3] the FDA lists the patents as scheduled for expiration on December 26, 2016, and August 29, 2017, respectively.[1]
Mechanism of action
Eletriptan is believed to reduce swelling of the blood vessels surrounding the brain. This swelling is associated with the head pain of a migraine attack. Eletriptan blocks the release of substances from nerve endings that cause more pain and other symptoms like nausea, and sensitivity to light and sound. It is thought that these actions contribute to relief of symptoms by eletriptan.
Eletriptan is a serotonin receptor agonist, specifically an agonist of certain 5-HT1 family receptors. Eletriptan binds with high affinity to the 5-HT[1B, 1D, 1F] receptors. It has a modest affinity to the 5-HT[1A, 1E, 2B, 7] receptors, and little to no affinity at the 5-HT[2A, 2C, 3, 4, 5A, 6] receptors.
Eletriptan has no significant affinity or pharmacological activity at adrenergic α1, α2, or β; dopaminergic D1 or D2; muscarinic; or opioid receptors. Eletriptan could be efficiently co-administrated with nitric oxide synthase (NOS's) inhibitors for the treatment of NOS-dependent diseases (US patent US 2007/0254940).
Two theories have been proposed to explain the efficacy of 5-HT1 receptor agonists in migraine. One theory suggests that activation of 5-HT1 receptors located on intracranial blood vessels, including those on the arteriovenous anastomoses, leads to vasoconstriction, which is correlated with the relief of migraine headache. The other hypothesis suggests that activation of 5-HT1 receptors on sensory nerve endings in the trigeminal system results in the inhibition of pro-inflammatory neuropeptide release.
Side effects
Common side effects include hypertension, tachycardia, headache, dizzyness, and symptoms similar to angina pectoris. Severe allergic reactions are rare.[4]
Contraindications
Eletriptan is contraindicated in patients with various diseases of the heart and circulatory system, such as angina pectoris, severe hypertension, and heart failure, as well as in patients that have had a stroke or heart attack. It is also contraindicated in severe renal or hepatic impairment.[4]
Interactions
The drug has a relatively low potential for interactions. Notably, it is unlikely to interact to a relevant extent with beta blockers, tricyclic antidepressants and SSRI type antidepressants. Strong inhibitors of the liver enzyme CYP3A4, such as erythromycin and ketoconazole, significantly increase blood plasma concentrations and half life of eletriptan. Ergot alkaloids add to the drug's hypertensive effect.[4]
Additional chemical names
- Merck Index: 3-[[(2R)-1-Methyl-2-pyrrolidinyl]methyl]-5-[2-(phenylsulfonyl)ethyl]-1H-indole
- 5-[2-(benzenesulfonyl)ethyl]-3-(1-methylpyrrolidin-2(R)-ylmethyl)-1H-indole
- (R)-5-[2-(phenylsulfonyl)ethyl]-3-[(1-methyl-2-pyrrolidinyl)methyl]-1H-indole
References
- 1 2 3 4 FDA AccessData entry for Eletriptan Hydrobromide, accessed March 10, 2010.
- ↑ U.S. Patent no. 5545644, John E. Macor & Martin J. Wythes, Indole Derivatives, August 13, 1996.
- ↑ U.S. Patent no. 6110940, Valerie Denise Harding, et al., Salts of an anti-migraine indole derivative, August 29, 2000.
- 1 2 3 Jasek, W, ed. (2007). Austria-Codex (in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. pp. 6984–8. ISBN 978-3-85200-181-4.
External links
- FDA label (December 2002)
- Physicians' Desk Reference entry for Relpax
- Medline Plus Drug Information for Eletriptan
- Pfizer Relpax site