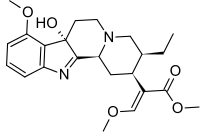
7-Hydroxymitragynine
 | |
| Names | |
|---|---|
| IUPAC name
Methyl (E)-2-[(2S,3S,7aS,12bS)-3-ethyl-7a-hydroxy-8-methoxy-2,3,4,6,7,12b-hexahydro-1H-indolo[2,3-a]quinolizin-2-yl]-3-methoxyprop-2-enoate | |
| Other names | |
| Identifiers | |
| 174418-82-7 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEMBL | ChEMBL61630 |
| ChemSpider | 23152144 |
| PubChem | 44301524 |
| UNII | 2T3TWA75R0 |
| |
| |
| Properties | |
| C23H30N2O5 | |
| Molar mass | 414.50 g·mol−1 |
| log P | 1.266 |
| Acidity (pKa) | 12.203 |
| Basicity (pKb) | 1.794 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
7-Hydroxymitragynine is a terpenoid indole alkaloid from the plant Mitragyna speciosa, commonly known as Kratom. In mice, it is orally active and has analgesic effects.[2]
7-Hydroxymitragynine is a partial agonist at the μ-opioid receptor[3] with a potency, calculated using pD (2) values, that is 30-fold higher than that of mitragynine and 17-fold higher than that of morphine, respectively.[4] As a G protein biased ligand at this receptor[5] it causes significantly less side effects than morphine,[6] like constipation, development of tolerance and withdrawal syndrome upon abstinence.[2] The O-acetyl ester (Acetoxy), 7-acetoxymitragynine has also been reported and found to be an active μ-opioid agonist.[7]

7-Acetoxymitragynine
See also
- Mitragynine
- Mitraphylline
- β-Prodine - molecule which overlays with 7-hydroxymitragynine's opioid QSAR
References
- 1 2 Chemical Abstracts Service: Columbus, OH, 2004; RN 174418-82-7 (accessed via SciFinder Scholar, version 2007.3; November 30, 2011)
- 1 2 Matsumoto K; Horie S; Ishikawa H; et al. (March 2004). "Antinociceptive effect of 7-hydroxymitragynine in mice: Discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa". Life Sci. 74 (17): 2143–55. doi:10.1016/j.lfs.2003.09.054. PMID 14969718.
- ↑ Takayama H; Ishikawa H; Kurihara M; Kitajima M; Aimi N; Ponglux D; Koyama F; Matsumoto K; Moriyama T; Yamamoto LT; Watanabe K; Murayama T; Horie S (April 2002). "Studies on the synthesis and opioid agonistic activities of mitragynine-related indole alkaloids: discovery of opioid agonists structurally different from other opioid ligands". J. Med. Chem. 45 (9): 1949–56. doi:10.1021/jm010576e. PMID 11960505.
- ↑ Horie S; Koyama F; Takayama H; et al. (March 2005). "Indole alkaloids of a Thai medicinal herb, Mitragyna speciosa, that has opioid agonistic effect in guinea-pig ileum". Planta Med. 71 (3): 231–6. doi:10.1055/s-2005-837822. PMID 15770543.
- ↑ Kruegel AC, Gassaway MM, Kapoor A, Váradi A, Majumdar S, Filizola M, Javitch JA, Sames D (2016). "Synthetic and Receptor Signaling Explorations of the Mitragyna Alkaloids: Mitragynine as an Atypical Molecular Framework for Opioid Receptor Modulators". J. Am. Chem. Soc. 138 (21): 6754–64. doi:10.1021/jacs.6b00360. PMID 27192616.
- ↑ Matsumoto K; Hatori Y; Murayama T; et al. (November 2006). "Involvement of mu-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa". Eur. J. Pharmacol. 549 (1–3): 63–70. doi:10.1016/j.ejphar.2006.08.013. PMID 16978601.
- ↑ Takayama H; Ishikawa H; Kurihara M; et al. (April 2002). "Studies on the synthesis and opioid agonistic activities of mitragynine-related indole alkaloids: discovery of opioid agonists structurally different from other opioid ligands". J. Med. Chem. 45 (9): 1949–56. doi:10.1021/jm010576e. PMID 11960505.
External links
- Takayama, H. (2004). "Chemistry and Pharmacology of Analgesic Indole Alkaloids from the Rubasceous Plant, Mitragyna speciosa" (pdf). Chem. Pharm. Bull. 52 (8): 916–928. doi:10.1248/cpb.52.916. PMID 15304982. - synthesis of 7-hydroxymitragynine from mitragynine
This article is issued from Wikipedia - version of the 9/22/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.