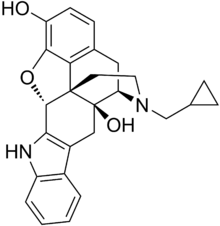
Naltrindole
Naltrindole|
|
| Clinical data |
|---|
Routes of
administration |
IV |
|---|
| ATC code |
none |
|---|
| Identifiers |
|---|
- 17-Cyclopropylmethyl-6,7-dehydro-4,5-epoxy -3,14-dihydroxy-6,7,2',3'-indolomorphinan
|
| CAS Number |
111555-53-4 |
|---|
| PubChem (CID) |
5497186 |
|---|
| IUPHAR/BPS |
1641 |
|---|
| ChemSpider |
4593753  Y Y |
|---|
| ChEMBL |
CHEMBL567175  Y Y |
|---|
| Chemical and physical data |
|---|
| Formula |
C26H26N2O3 |
|---|
| Molar mass |
414.496 g/mol |
|---|
| 3D model (Jmol) |
Interactive image |
|---|
Oc4c3O[C@H]7c2c(c1ccccc1n2)C[C@@]6(O)[C@H]5N(CC[C@@]67c3c(cc4)C5)CC8CC8
|
InChI=1S/C26H26N2O3/c29-19-8-7-15-11-20-26(30)12-17-16-3-1-2-4-18(16)27-22(17)24-25(26,21(15)23(19)31-24)9-10-28(20)13-14-5-6-14/h1-4,7-8,14,20,24,27,29-30H,5-6,9-13H2/t20-,24+,25+,26-/m1/s1  Y YKey:WIYUZYBFCWCCQJ-IFKAHUTRSA-N  Y Y
|
| (verify) |
|---|
Naltrindole is a highly potent, highly selective delta opioid receptor antagonist used in biomedical research. In May 2012 a paper was published in Nature with the structure of naltrindole in complex with the mouse δ-opioid G-protein coupled receptor, solved by X-ray crystallography.[1]
Drug design
Since peptide compounds are unable to cross the blood–brain barrier, researchers developed naltrindole to be a non-peptide antagonist analog of the delta-preferring endogenous opiate enkephalin. Enkephalin contains an aromatic phenyl group on its Phe4 residue, which was hypothesized to be the "address" sequence responsible for the opiate's delta opioid receptor affinity.[2] Thus, attachment of a phenyl-containing indole molecule to the C-ring of naltrexone's morphinan base successfully produced a drug with the high receptor affinity of naltrexone, but which binds almost exclusively to the delta opioid receptor.[3]
References
- ↑ Granier, S.; Manglik, A.; Kruse, A. C.; Kobilka, T. S.; Thian, F. S.; Weis, W. I.; Kobilka, B. K. (2012). "Structure of the δ-opioid receptor bound to naltrindole". Nature. 485 (7398): 400–404. doi:10.1038/nature11111. PMC 3523198
 . PMID 22596164.
. PMID 22596164. - ↑ Lipkowski, AW; Tam, SW; Portoghese, PS (Jul 1986). "Peptides as receptor selectivity modulators of opiate pharmacophores". Journal of Medicinal Chemistry. 29 (7): 1222–5. doi:10.1021/jm00157a018. PMID 2879914.
- ↑ Portoghese, PS; Sultana, M; Takemori, AE (Jan 1988). "Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist". European Journal of Pharmacology. 146 (1): 185–6. doi:10.1016/0014-2999(88)90502-X. PMID 2832195.
|
|---|
|
| MOR |
- PAMs: BMS-986121
- BMS-986122
|
|---|
|
| DOR | |
|---|
|
| KOR |
- Agonists: 6'-GNTI
- 8-CAC
- 18-MC
- 14-Methoxymetopon
- β-Chlornaltrexamine
- β-Funaltrexamine
- Adrenorphin (metorphamide)
- Akuuamicine
- Alazocine
- Allomatrine
- Asimadoline
- BAM-12P
- BAM-18P
- BAM-22P
- Big dynorphin
- Bremazocine
- BRL-52537
- Butorphan
- Butorphanol
- BW-373U86
- Cebranopadol
- Ciprefadol
- CR665
- Cyclazocine
- Cyclorphan
- Cyprenorphine
- Diamorphine (heroin)
- Diacetylnalorphine
- Difelikefalin
- Dihydroetorphine
- Dihydromorphine
- Diprenorphine
- Dynorphin A
- Dynorphin B (rimorphin)
- Eluxadoline
- Enadoline
- Eptazocine
- Erinacine E
- Ethylketazocine
- Etorphine
- Fedotozine
- Fentanyl
- Gemazocine
- GR-89696
- GR-103545
- Hemorphin-4
- Herkinorin
- HS665
- Hydromorphone
- HZ-2
- Ibogaine
- ICI-199,441
- ICI-204,448
- Ketamine
- Ketazocine
- Laudanosine
- Leumorphin (dynorphin B-29)
- Levallorphan
- Levomethorphan
- Levorphanol
- Lexanopadol
- Lofentanil
- LPK-26
- Lufuradom
- Matrine
- MB-1C-OH
- Menthol
- Metazocine
- Metkefamide
- Mianserin
- Mirtazapine
- Morphine
- Moxazocine
- MR-2034
- N-MPPP
- Nalbuphine
- Nalbuphine sebacate
- NalBzOH
- Nalfurafine
- Nalmefene
- Nalodeine (N-allylnorcodeine)
- Nalorphine
- Naltriben
- Niravoline
- Norbuprenorphine
- Norbuprenorphine-3-glucuronide
- Noribogaine
- Norketamine
- O-Desmethyltramadol
- Oripavine
- Oxilorphan
- Oxycodone
- Pentazocine
- Pethidine (meperidine)
- Phenazocine
- Proxorphan
- Racemethorphan
- Racemorphan
- RB-64
- Salvinorin A (salvia)
- Salvinorin B ethoxymethyl ether
- Salvinorin B methoxymethyl ether
- Samidorphan
- SKF-10047
- Spiradoline (U-62,066)
- TH-030418
- Thienorphine
- Tifluadom
- Tricyclic antidepressants (e.g., amitriptyline, desipramine, imipramine, nortriptyline)
- U-50,488
- U-54,494A
- U-69,593
- Xorphanol
|
|---|
|
| NOP | |
|---|
|
| Unsorted | |
|---|
|
| Others |
- Others: Kyotorphin (met-enkephalin releaser/degradation stabilizer)
|
|---|
|
See also: Peptide receptor modulators |
 . PMID 22596164.
. PMID 22596164.