Epicatechin gallate
Epicatechin gallate
 |
| Names |
| IUPAC name
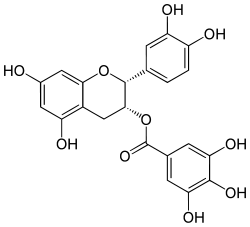
[(2R,3R)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate |
| Other names
|
| Identifiers |
| |
1257-08-5  Y Y |
| 3D model (Jmol) |
Interactive image |
| ChEBI |
CHEBI:70255  N N |
| ChEMBL |
ChEMBL36327  N N |
| ChemSpider |
97034  N N |
| ECHA InfoCard |
100.116.252 |
| PubChem |
107905 |
InChI=1S/C22H18O10/c23-11-6-14(25)12-8-19(32-22(30)10-4-16(27)20(29)17(28)5-10)21(31-18(12)7-11)9-1-2-13(24)15(26)3-9/h1-7,19,21,23-29H,8H2/t19-,21-/m1/s1  N NKey: LSHVYAFMTMFKBA-TZIWHRDSSA-N  N NInChI=1/C22H18O10/c23-11-6-14(25)12-8-19(32-22(30)10-4-16(27)20(29)17(28)5-10)21(31-18(12)7-11)9-1-2-13(24)15(26)3-9/h1-7,19,21,23-29H,8H2/t19-,21-/m1/s1 Key: LSHVYAFMTMFKBA-TZIWHRDSBY
|
O=C(O[C@@H]2Cc3c(O[C@@H]2c1ccc(O)c(O)c1)cc(O)cc3O)c4cc(O)c(O)c(O)c4
|
| Properties |
| |
C22H18O10 |
| Molar mass |
442.37 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
 N verify (what is N verify (what is  Y Y N ?) N ?) |
| Infobox references |
|
|
Epicatechin gallate (ECG) is a flavan-3-ol, a type of flavonoid, present in green tea.[1] It is also reported in buckwheat[2] and in grape.[3]
The tea component epicatechin gallate is being researched because in vitro experiments showed it can reverse methicillin resistance in bacteria like Staphylococcus aureus.[1] If confirmed, this means the combined intake of a tea extract containing this component might also enhance the effectiveness of methicillin treatment against some resistant bacteria in vivo.
Epicatechin, as well as many other flavonoids, has been found to act as a non-selective antagonist of the opioid receptors, albeit with somewhat low affinity.[4]
References
- 1 2 Shiota, S; Shimizu, M; Mizushima, T; Ito, H; Hatano, T; Yoshida, T; Tsuchiya, T (1999). "Marked reduction in the minimum inhibitory concentration (MIC) of beta-lactams in methicillin-resistant Staphylococcus aureus produced by epicatechin gallate, an ingredient of green tea (Camellia sinensis)". Biological & Pharmaceutical Bulletin. 22 (12): 1388–90. doi:10.1248/bpb.22.1388. PMID 10746177.
- ↑ Danila, Ana-Maria; Kotani, Akira; Hakamata, Hideki; Kusu, Fumiyo (2007). "Determination of Rutin, Catechin, Epicatechin, and Epicatechin Gallate in BuckwheatFagopyrum esculentumMoench by Micro-High-Performance Liquid Chromatography with Electrochemical Detection". Journal of Agricultural and Food Chemistry. 55 (4): 1139–43. doi:10.1021/jf062815i. PMID 17253718.
- ↑ Souquet, Jean-Marc; Cheynier, Véronique; Brossaud, Franck; Moutounet, Michel (1996). "Polymeric proanthocyanidins from grape skins". Phytochemistry. 43 (2): 509–512. doi:10.1016/0031-9422(96)00301-9.
- ↑ Katavic PL, Lamb K, Navarro H, Prisinzano TE (August 2007). "Flavonoids as opioid receptor ligands: identification and preliminary structure-activity relationships". J. Nat. Prod. 70 (8): 1278–82. doi:10.1021/np070194x. PMC 2265593
 . PMID 17685652.
. PMID 17685652.
See also
|
|---|
|
Receptor
(ligands) | |
- Antibodies: Brizantin (Бризантин)
- Dietressa (Диетресса)
|
|---|
| |
- Agonists: 2-AG
- 2-AGE (noladin ether)
- 3,3'-Diindolylmethane
- 4-O-Methylhonokiol
- α-Amyrin
- β-Amyrin
- A-796,260
- A-834,735
- A-836,339
- AM-1172
- AM-1221
- AM-1235
- AM-1241
- AM-2232
- Anandamide
- AZ-11713908
- Cannabinol
- Caryophyllene
- CB-13
- CBS-0550
- CP-55,940
- GW-405,833 (L-768,242)
- GW-842,166X
- HU-308
- JTE 7-31
- JWH-007
- JWH-015
- JWH-018
- JWH-73
- JWH-133
- L-759,633
- L-759,656
- Magnolol
- MDA-19
- Nabitan
- NADA
- PF-03550096
- S-444,823
- SER-601
- Serinolamide A
- UR-144
- Tedalinab
- THC (dronabinol)
- THCV
- Tetrahydromagnolol
- Virodhamine
|
|---|
| | |
|---|
| | |
|---|
| | |
|---|
|
|---|
|
Transporter
(modulators) | |
|---|
|
Enzyme
(modulators) | | |
|---|
| | |
|---|
| |
- Inhibitors: JZP-169
- JZP-430
- KT182
- KT185
- KT195
- KT203
- LEI-106
- ML294
- ML295
- ML296
- UCM710
- WWL-70
|
|---|
| | |
|---|
|
|---|
|
| Others |
- Others: 2-PG (directly potentiates activity of 2-AG at CB1 receptor)
- ARN-272 (FAAH-like anandamide transporter inhibitor)
|
|---|
|
- See also: Cannabinoids (cannabinoids by structure)
|
|
|---|
|
| MOR |
- PAMs: BMS-986121
- BMS-986122
|
|---|
|
| DOR | |
|---|
|
| KOR |
- Agonists: 6'-GNTI
- 8-CAC
- 18-MC
- 14-Methoxymetopon
- β-Chlornaltrexamine
- β-Funaltrexamine
- Adrenorphin (metorphamide)
- Akuuamicine
- Alazocine
- Allomatrine
- Asimadoline
- BAM-12P
- BAM-18P
- BAM-22P
- Big dynorphin
- Bremazocine
- BRL-52537
- Butorphan
- Butorphanol
- BW-373U86
- Cebranopadol
- Ciprefadol
- CR665
- Cyclazocine
- Cyclorphan
- Cyprenorphine
- Diamorphine (heroin)
- Diacetylnalorphine
- Difelikefalin
- Dihydroetorphine
- Dihydromorphine
- Diprenorphine
- Dynorphin A
- Dynorphin B (rimorphin)
- Eluxadoline
- Enadoline
- Eptazocine
- Erinacine E
- Ethylketazocine
- Etorphine
- Fedotozine
- Fentanyl
- Gemazocine
- GR-89696
- GR-103545
- Hemorphin-4
- Herkinorin
- HS665
- Hydromorphone
- HZ-2
- Ibogaine
- ICI-199,441
- ICI-204,448
- Ketamine
- Ketazocine
- Laudanosine
- Leumorphin (dynorphin B-29)
- Levallorphan
- Levomethorphan
- Levorphanol
- Lexanopadol
- Lofentanil
- LPK-26
- Lufuradom
- Matrine
- MB-1C-OH
- Menthol
- Metazocine
- Metkefamide
- Mianserin
- Mirtazapine
- Morphine
- Moxazocine
- MR-2034
- N-MPPP
- Nalbuphine
- Nalbuphine sebacate
- NalBzOH
- Nalfurafine
- Nalmefene
- Nalodeine (N-allylnorcodeine)
- Nalorphine
- Naltriben
- Niravoline
- Norbuprenorphine
- Norbuprenorphine-3-glucuronide
- Noribogaine
- Norketamine
- O-Desmethyltramadol
- Oripavine
- Oxilorphan
- Oxycodone
- Pentazocine
- Pethidine (meperidine)
- Phenazocine
- Proxorphan
- Racemethorphan
- Racemorphan
- RB-64
- Salvinorin A (salvia)
- Salvinorin B ethoxymethyl ether
- Salvinorin B methoxymethyl ether
- Samidorphan
- SKF-10047
- Spiradoline (U-62,066)
- TH-030418
- Thienorphine
- Tifluadom
- Tricyclic antidepressants (e.g., amitriptyline, desipramine, imipramine, nortriptyline)
- U-50,488
- U-54,494A
- U-69,593
- Xorphanol
|
|---|
|
| NOP | |
|---|
|
| Unsorted | |
|---|
|
| Others |
- Others: Kyotorphin (met-enkephalin releaser/degradation stabilizer)
|
|---|
|
See also: Peptide receptor modulators |
|
|---|
|
| Flavan-3-ols | |
|---|
|
| O-methylated flavan-3ols |
- Meciadanol (3-O-methylcatechin)
- Ourateacatechin (4′-O-methyl-(−)-epigallocatechin)
|
|---|
|
| Glycosides |
- Arthromerin A (Afzelechin-3-O-β-D-xylopyranoside)
- Arthromerin B (Afzelechin-3-O-β-D-glucopyranoside)
- Catechin-3-O-glucoside
- Catechin-3'-O-glucoside
- Catechin-4'-O-glucoside
- Catechin-5-O-glucoside
- Catechin-7-O-glucoside
- (+)-Catechin 7-O-β-D-xylopyranoside
- Epicatechin-3′-O-glucoside
- Glochiflavanoside A, B, C D
- Polydine ((+)-catechin 7-0-α-L-arabinoside)
- Symplocoside (3’-O-methyl-(-)-epicatechin 7-O-β-D-glucopyranoside)
|
|---|
|
| Acetylated | |
|---|
|
| Misc. | |
|---|

 . PMID 17685652.
. PMID 17685652.