Orbitofrontal cortex
| Orbitofrontal cortex | |
|---|---|
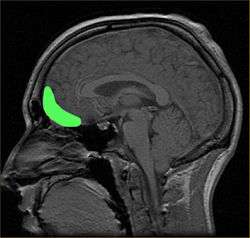 Approximate location of the OFC shown on a sagittal MRI | |
 Orbital surface of left frontal lobe. | |
| Details | |
| Identifiers | |
| Latin | Cortex orbitofrontalis |
| NeuroNames | hier-73 |
| NeuroLex ID | Orbital frontal cortex |
The orbitofrontal cortex (OFC) is a prefrontal cortex region in the frontal lobes in the brain which is involved in the cognitive processing of decision-making. In non-human primates it consists of the association cortex areas Brodmann area 11, 12 and 13; in humans it consists of Brodmann area 10, 11 and 47[1]
The OFC is considered anatomically synonymous with the ventromedial prefrontal cortex.[2] Therefore, the region is distinguished due to the distinct neural connections and the distinct functions it performs.[3] It is defined as the part of the prefrontal cortex that receives projections from the magnocellular, medial nucleus of the mediodorsal thalamus, and is thought to represent emotion and reward in decision making.[4] It gets its name from its position immediately above the orbits in which the eyes are located. Considerable individual variability has been found in the OFC of both humans and non-human primates. A related area is found in rodents.[5]
Functions of the human orbitofrontal cortex
The human OFC is among the least-understood regions of the human brain; but it has been proposed that the OFC is involved in sensory integration, in representing the affective value of reinforcers, and in decision-making and expectation.[1] In particular, the OFC seems to be important in signaling the expected rewards/punishments of an action given the particular details of a situation.[6] In doing this, the brain is capable of comparing the expected reward/punishment with the actual delivery of reward/punishment, thus, making the OFC critical for adaptive learning. This is supported by research in humans, non-human primates, and rodents. Human research has foccused on neuroimaging research in healthy participants and neuropsychology research in patients with damage to discrete parts of the OFC. Research at the University of Leipzig shows that the human OFC is activated during intuitive coherence judgements.[7]
Connectivity of the orbitofrontal cortex
Tracer studies in monkeys have shown that the orbitofrontal cortex shares extensive connections with other association cortices, primary sensory and association cortices, limbic systems, and other subcortical areas. Corticocortical connections include extensive local projections to and from other prefrontal regions, as well as with motor, limbic, and sensory cortices. Areas projecting to motor areas are densely interconnected with other prefrontal cortical regions, reflecting integration for executive motor control.[8]
Sensory cortices additionally share highly complex reciprocal connections with the orbitofrontal cortex. All sensory modalities are represented in connections with the orbitofrontal cortex, including extensive innervation from areas associated with olfaction and gustatory somatic responses.[9] Somatosensory cortices including primary areas 1 and 2, particularly in areas associated with innervation of the hand and trigeminal complex, indicating the importance of the orbitofrontal cortex in face and hand sensation.[8]
Functionally distinct pathways for auditory processing in the orbitofrontal cortex include a rostral stream associated with phonetic processing, and a more caudal stream terminating just posterior to the orbitofrontal cortex in the periarcuate prefrontal cortex associated with auditory-spatial processing, though these connections share extensive overlap. Both ventral and dorsal visual streams share connections with orbitofrontal cortical areas, including rich projections to and from the superior temporal pole, important for integration of spatial and object processing.[8]
Connectivity of the orbitofrontal cortex with limbic areas includes reciprocal projections to granular, dysgranular, and agranular insular cortex, parahippocampal regions, and the hippocampus, particularly CA1 regions in a rostral-to caudal gradient.[8] The orbitofrontal cortex additionally shares extensive reciprocal connections with the amygdala, and direct and indirect connections to the hypothalamus.[10]
Additional subcortical projections are shared between the striatum, particularly ventral reward-related areas,.[1][11] Connectivity with thalamic and periaqueductal grey areas further suggests a role for the orbitofrontal cortex in both inhibitory and excitatory regulation of autonomic function.[8] Parallel processing loops in connectivity between cortico-striatal networks seem to be involved in the processing of goal-directed and habitual action, whereas cortico-limbic connectivity seems to be of prime importance for action selection, implicating the basolateral amygdala, and the integration of information into behavioral output.[12]
The connections between orbitofrontal cortex and amygdala play a notable role in emotional decision making process. These connections contribute in modulating the associative learning process and emotion regulation in amygdala.[13]
Though invasive tracer studies are largely not possible in humans, diffusion tensor imaging (DTI) tractography studies have also been used to map the connectivity of the orbitofrontal cortex to cortical and subcortical brain structures. Connections in the human orbitofrontal cortex follow a conserved pattern, similar to what is shown in tracer studies in rhesus macaques, but with a distinct pattern of connectivity with regions of the striatum.[14]
Clinical applications of OFC connectivity disruptions
When OFC connections are disrupted, a number of cognitive, behavioral, and emotional consequences may arise. Research supports that the main disorders associated with dysregulated OFC connectivity/circuitry center around decision-making, emotion regulation, and reward expectation.[15][16][17] A recent multi-modal human neuroimaging study shows disrupted structural and functional connectivity of the OFC with the subcortical limbic structures (e.g., amygdala or hippocampus) and other frontal regions (e.g., dorsoal prefrontal cortex or anterior cingulate cortex) correlates with abnormal OFC affect (e.g., fear) processing in clinically anxious adults.[18]
One clear extension of problems with decision-making is drug addiction/substance dependence, which can result from disruption of the striato-thalamo-orbitofrontal circuit.[17][15][19] Attention deficit hyperactivity disorder (ADHD) has also been implicated in dysfunction of neural reward circuitry controlling motivation, reward, and impulsivity, including OFC systems.[16] Other disorders of executive functioning and impulse control may be affected by OFC circuitry dysregulation, such as obsessive–compulsive disorder and trichotillomania[20][21][22]
Some dementias are also associated with OFC connectivity disruptions. The behavioral variant of frontotemporal dementia[23] is associated with neural atrophy patterns of white and gray matter projection fibers involved with OFC connectivity.[24] Finally, some research suggests that later stages of Alzheimer’s Disease be impacted by altered connectivity of OFC systems.[22]
Medial and lateral distinction
The OFC has been cytoarchitectonically and connectionally subdivided into a medial and a lateral portion. The medial portion has its strongest connections with the hippocampus and associated areas of the cingulate, retrosplenial and entorhinal cortices, anterior thalamus and septal diagonal band. The lateral portion can be further subdivided into three sectors. The most caudal sector is characterized by strong connections with the amygdala, midline thalamus, non-isocortical insula and temporal pole. The most anterior sector has more pronounced connections with the granular insula, association cortex, mediodorsal thalamus, inferior parietal lobule and dorsolateral PFC.[25]
It is suggested that the medial OFC is involved in making stimulus-reward associations and with the reinforcement of behavior, while the lateral OFC is involved in stimulus-outcome associations and the evaluation and possibly reversal of behavior.[26] Activity in the lateral OFC is found, for example, when subjects encode new expectations about punishment and social reprisal.[27][28] It is also found when suppressing negative emotions, especially in approach-avoidance situations, such as the game of chicken.[29] The lateral OFC plays an important role in conflict resolution and damage to this area results in both inappropriate displays of anger and inappropriate responses to the anger of others. For example, subjects with damage to the left lateral OFC have been found to be defensive and to present themselves in an "angelic light".[30] Low volume in this area has also been correlated with experiencing "fear of God".[31] On the other hand, subjects with greater volume in this area have been found to score higher on the Mach IV test measuring Machiavellian personality traits[32] and activity in this region has generally been connected with Machiavellian thinking .[33]
In one study, adults who were classified as "high-reactive" as children, meaning shy and inhibited, were found to have greater cortical thickness in the right ventromedial prefrontal cortex, while adults who were classified as "low-reactive", meaning outgoing and uninhibited, were found to have greater thickness in the left lateral orbitofrontal cortex.[34]
Orbitofrontal cortex and addiction
Involvement of OFC is often implicated in addictive behavior in addition to the nucleus accumbens and amygdala . The striato-thalamo-orbitofrontal circuit of the OFC has been implicated in the development of addictive behavior via dopaminergic activation of reward circuits as supported by brain imaging studies. The OFC has been associated with compulsive behavior and repetitive behavior, as well as with drive;[35] in drug dependent individuals, disruption of the striato-thalamo- orbitofrontal circuit leads to compulsive behavior and increased motivation to take the drug.
Addicted individuals show deficits in orbitofrontal, striatal, and thalamic regions.[36] Conscious and unconscious components are hypothesized to serve as mechanisms responsible for the maintenance of drug addiction: conscious mechanisms involve craving associated with loss of control and unconscious elements include anticipated conditioned responses to a drug and impulsivity.[36]
Brain imaging studies show that during cocaine withdrawal, metabolism is increased in the OFC and that this is proportional to drug craving. In contrast, during protracted (up to 3–4 months) withdrawal cocaine abusers show reduced activity in the OFC compared to healthy controls.[37][38]
Similarly, in alcoholics, during withdrawal there is decreased activity in the OFC (compared to the OFCs of healthy controls)[36] but, in addition, detoxified alcoholics have significantly lower levels of benzodiazepine receptors in the OFC (compared with healthy controls).[39] Hypoactivity in the OFC of alcoholics is also supported by blunted metabolism in the OFC to response to both serotonogenic and GABA- ergic agents.[36]
Anatomy of OFC and addiction
The OFC projects and is neuronanatomically connected (via the mediodorsal nucleus of the thalamus) to the nucleus accumbens - which is associated with the reinforcing effects of drug administration.[40][41][42] The nucleus accumbens projects back to the OFC,[43] as do dopamine cells in the ventral tegmental area (VTA)[44] - the latter being associated with the positive, reinforcing effects of drugs. Limbic regions including the amygdala, hippocampus and cingulate gyrus also project to the OFC via direct and indirect pathways[41][45] and it would appear that the OFC is not only the target for reinforcing drug effects but also serves to integrate information from the limbic system, modulating the response of the limbic areas to drugs of abuse (and their rewarding effects).[36]
OFC and addiction in animal studies
(For a review see Porrino & Lyons, 2000.) Stimulating the OFC in laboratory animals results in drug self- administration.[36] In animal studies the OFC is hypothesized to not only be associated with the response to reward, but also to respond and adjust animal behavior when the rewarding properties of the reinforcement change[46] - as well as learning the association between stimulus and reward. The damage to the OFC results in deficits in reversal of stimulus reinforcement in which an animal perseverates on a behavior and fails to extinguish a behavior.[47] This perseveration and inability to extinguish a behavior can be related to drug administration in substance abuse and substance dependence where individuals compulsively self-administer a drug even with drastic decrease of reinforcing effects of that drug and tolerance to the pleasurable effects and in the presence of adverse consequences of drug use. Rats who are reintroduced to an environment in which they used cocaine experience activation of the OFC.[48] In addition, in rats, repeated alcohol use causes degeneration of the OFC.[49]
Neuroimaging research in healthy participants
Using functional magnetic resonance imaging (fMRI) to image the human OFC is a challenge, because this brain region is in proximity to the air-filled sinuses. This means that signal dropout, geometric distortion and susceptibility artifacts are common when using EPI at higher magnetic field strengths. Extra care is therefore recommended for obtaining a good signal from the orbitofrontal cortex, and a number of strategies have been devised, such as automatic shimming at high static magnetic field strengths.[50]
The published neuroimaging studies have found that the reward value, the expected reward value, and even the subjective pleasantness of foods and other reinforcers are represented in the OFC. A large meta-analysis of the existing neuroimaging evidence demonstrated that activity in medial parts of the OFC is related to the monitoring, learning, and memory of the reward value of reinforcers, whereas activity in lateral OFC is related to the evaluation of punishers, which may lead to a change in ongoing behaviour.[51] Similarly, a posterior-anterior distinction was found with more complex or abstract reinforcers (such as monetary gain and loss) being represented more anteriorly in the orbitofrontal cortex than less-complex reinforcers such as taste. It has even been proposed that the human OFC has a role in mediating subjective hedonic experience.[1]
The orbitofrontal cortex (OFC) represents the main neocortical target of primary olfactory cortex. In non-human primates, the olfactory neocortex is situated along the basal surface of the caudal frontal lobes, encompassing agranular and dysgranular OFC medially and agranular insula laterally, where this latter structure wraps onto the posterior orbital surface. Direct afferent inputs arrive from most primary olfactory areas, including piriform cortex, amygdala, and entorhinal cortex, in the absence of an obligatory thalamic relay.[52]
Neuropsychology research in patients
Visual discrimination test
This has two components. In the first component, "reversal learning", participants are presented with one of two pictures, A and B. They learn that they will be rewarded if they press a button when picture A is displayed, but punished if they press the button when picture B is displayed. Once this rule has been established, the rule swaps. In other words, now it is correct to press the button for picture B, not picture A. Most healthy participants pick up on this rule reversal almost immediately, but patients with OFC damage continue to respond to the original pattern of reinforcement, although they are now being punished for persevering with it. Rolls et al.[53] noted that this pattern of behaviour is particularly unusual given that the patients reported that they understood the rule.
The second component of the test is "extinction". Again, participants learn to press the button for picture A but not picture B. However this time, instead of the rules reversing, the rule changes altogether. Now the participant will be punished for pressing the button in response to either picture. The correct response is not to press the button at all, but people with OFC dysfunction find it difficult to resist the temptation to press the button despite being punished for it.
Iowa gambling task
A simulation of real life decision-making, the Iowa gambling task is widely used in cognition and emotion research.[54] Participants are presented with four virtual decks of cards on a computer screen. They are told that each time they choose a card they will win some game money. Every so often, however, when they choose a card they will lose some money. They are told that the aim of the game is to win as much money as possible. The task is meant to be opaque, that is, participants are not meant to consciously work out the rule, and they are supposed to choose cards based on their "gut reaction." Two of the decks are "bad decks," which means that, over a long enough time, they will make a net loss; the other two decks are "good decks" and will make a net gain over time.
Most healthy participants sample cards from each deck, and after about 40 or 50 selections are fairly good at sticking to the good decks. Patients with OFC dysfunction, however, continue to perseverate with the bad decks, sometimes even though they know that they are losing money overall. Concurrent measurement of galvanic skin response shows that healthy participants show a "stress" reaction to hovering over the bad decks after only 10 trials, long before conscious sensation that the decks are bad. By contrast, patients with OFC dysfunction never develop this physiological reaction to impending punishment. Bechara and his colleagues explain this in terms of the somatic marker hypothesis. The Iowa gambling task is currently being used by a number of research groups using fMRI to investigate which brain regions are activated by the task in healthy volunteers as well as clinical groups with conditions such as schizophrenia and obsessive compulsive disorder.
Faux pas test
This is a series of vignettes recounting a social occasion during which someone said something that should not have been said, or an awkward occurrence. The participant's task is to identify what was said that was awkward, why it was awkward, how people would have felt in reaction to the faux pas and to a factual control question. Although first designed for use in people on the autism spectrum,[55] the test is also sensitive to patients with OFC dysfunction, who cannot judge when something socially awkward has happened despite appearing to understand the story perfectly well.
Consequences of damage to the OFC
Destruction of the OFC through acquired brain injury typically leads to a pattern of disinhibited behaviour. Examples include swearing excessively, hypersexuality, poor social interaction, compulsive gambling, drug use (including alcohol and tobacco), and poor empathising ability. Disinhibited behaviour by patients with some forms of frontotemporal dementia is thought to be caused by degeneration of the OFC.[56]
See also
- BELBIC
- Eadweard Muybridge
- Social emotional learning
- Obsessive-compulsive disorder
- Phineas Gage
- Witzelsucht
- Brain Emotional Learning Inspired Models
Additional images
 Orbital gyrus shown in red.
Orbital gyrus shown in red. Medial surface of cerebral cortex - gyri
Medial surface of cerebral cortex - gyri Basal surface of cerebrum. Orbital gyrus shown in red.
Basal surface of cerebrum. Orbital gyrus shown in red.
References
- 1 2 3 4 Kringelbach M. L. (2005). "The orbitofrontal cortex: linking reward to hedonic experience". Nature Reviews Neuroscience. 6: 691–702. doi:10.1038/nrn1747.
- ↑ Phillips, LH., MacPherson, SE. & Della Sala, S. (2002). 'Age, cognition and emotion: the role of anatomical segregation in the frontal lobes: the role of anatomical segregation in the frontal lobes'. in J Grafman (ed.), Handbook of Neuropsychology: the frontal lobes. Elsevier Science, Amsterdam, pp. 73-98.
- ↑ Barbas H, Ghashghaei H, Rempel-Clower N, Xiao D (2002) Anatomic basis of functional specialization in prefrontal cortices in primates. In: Handbook of Neuropsychology (Grafman J, ed), pp 1-27. Amsterdam: Elsevier Science B.V.
- ↑ Fuster, J.M. The Prefrontal Cortex, (Raven Press, New York, 1997).
- ↑ Uylings HB, Groenewegen HJ, Kolb B (2003). "Do rats have a prefrontal cortex?". Behav Brain Res. 146 (1-2): 3–17. doi:10.1016/j.bbr.2003.09.028. PMID 14643455.
- ↑ Schoenbaum G, Takahashi Y, Liu T, McDannald M (2011). "Does the orbitofrontal cortex signal value?". Annals of the New York Academy of Sciences. 1239: 87–99. doi:10.1111/j.1749-6632.2011.06210.x.
- ↑ Volz KG, Rübsamen R, von Cramon DY (September 2008). "Cortical regions activated by the subjective sense of perceptual coherence of environmental sounds: a proposal for a neuroscience of intuition". Cogn Affect Behav Neurosci. 8 (3): 318–28. doi:10.3758/CABN.8.3.318. PMID 18814468.
- 1 2 3 4 5 Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez (2000). "The anatomical connections of the macaque monkey orbitofrontal cortex". Cerebral Cortex. 10: 220–42. doi:10.1093/cercor/10.3.220.
- ↑ Rolls E. T. "The orbitofrontal cortex and reward". Cerebral Cortex. 20: 284–294.
- ↑ Barbas H (2007). "Flow of information for emotions through temporal and orbitofrontal pathways". Journal of Anatomy. 211: 237–49. doi:10.1111/j.1469-7580.2007.00777.x.
- ↑ Schultz W, Tremblay L (2006). Involvement of primate orbitofrontal neurons in rewards, uncertainty, and learning. In Zald DH and Rauch SL (Eds.) The Orbitofrontal Cortex. Oxford: University Press.
- ↑ Balleine BW, O'Doherty JP (2010). "Human and Rodent Homologies in Action Control: Corticostriatal Determinants of Goal-Directed and Habitual Action". Neuropsychopharmacology Reviews. 35: 48–69. doi:10.1038/npp.2009.131.
- ↑ Barbas, H. (2007). "Specialized elements of orbitofrontal cortex in primates.". doi:10.1196/annals.1401.015.
- ↑ Lehericy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim DS (2004). "Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans". Annals of Neurology. 55 (4): 522–529.
- 1 2 Paulus M. P.; Hozack N. E.; Zauscher B. E.; Frank L.; Brown G. G.; Braff D. L.; Schuckit M. A. (2002). "Behavioral and Functional Neuroimaging Evidence for Prefrontal Dysfunction in Methamphetamine-Dependent Subjects". Neuropsychopharmacology. 1: 53–63.
- 1 2 Toplak M. E.; Jain U.; Tannock R. (2005). "Executive and motivational processes in adolescents with Attention-Deficit-Hyperactivity Disorder (ADHD)". Behavioral and Brain Functions. 1: 8–20.
- 1 2 Verdejo-Garcia A.; Bechara A.; Recknor E. C.; Perez-Garcia M. (2006). "Executive dysfunction in substance dependent individuals during drug use and abstinence: An examination of the behavioral, cognitive and emotional correlates of addiction". Journal of the International Neuropsychological Society. 12: 405–415. doi:10.1017/s1355617706060486.
- ↑ Cha, Jiook; Greenberg, Tsafrir; Carlson, Joshua M.; DeDora, Daniel J.; Hajcak, Greg; Mujica-Parodi, Lilianne R. (2014-03-12). "Circuit-Wide Structural and Functional Measures Predict Ventromedial Prefrontal Cortex Fear Generalization: Implications for Generalized Anxiety Disorder". The Journal of Neuroscience. 34 (11): 4043–4053. doi:10.1523/JNEUROSCI.3372-13.2014. ISSN 0270-6474. PMID 24623781.
- ↑ Volkow N.D.; Fowler J.S. (2000). "Addiction a disease of compulsion and drive: involvement of the orbitofrontal cortex". Cerebral Cortex. 10: 318–325. doi:10.1093/cercor/10.3.318.
- ↑ Chamberlain S. R.; Odlaug B. L.; Boulougouris V.; Fineberg N. A.; Grant J. E. (2009). "Trichotillomania: Neurobiology and treatment". Neuroscience and Biobehavioral Reviews. 3: 831–842.
- ↑ Menzies L. (2008). "Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: The orbitofronto-striatal model revisited". Neuroscience and Biobehavioral Reviews. 32: 525–549. doi:10.1016/j.neubiorev.2007.09.005.
- 1 2 Tekin S.; Cummings J. L. (2002). "Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update". Journal of Psychosomatic Research. 53: 647–654. doi:10.1016/s0022-3999(02)00428-2.
- ↑ Rahman S.; Sahakian B. J.; Hodges J. R.; Rogers R. D.; Robbins T. W. (1999). "Specific cognitive deficits in early behavioural variant frontotemporal dementia". Brain. 122: 1469–1493. doi:10.1093/brain/122.8.1469.
- ↑ Seeley W. W.; Crawford R.; Rascovsky K.; Kramer J. H.; Weiner M.; Miller B. L.; Gorno-Tempini L. (2008). "Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia". Archives of Neurology. 65: 249–255. doi:10.1001/archneurol.2007.38.
- ↑ Elliott R.; Dolan R. J.; Frith C. D. (2000). "Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies". Cerebral cortex. 10 (3): 308–317. doi:10.1093/cercor/10.3.308.
- ↑ Walton M. E.; Behrens T. E.; Buckley M. J.; Rudebeck P. H.; Rushworth M. F. (2010). "Separable learning systems in the macaque brain and the role of orbitofrontal cortex in contingent learning". Neuron. 65 (6): 927–939. doi:10.1016/j.neuron.2010.02.027.
- ↑ Campbell-Meiklejohn, D. K., Kanai, R., Bahrami, B., Bach, D. R., Dolan, R. J., Roepstorff, A., & Frith, C. D. (2012). Structure of orbitofrontal cortex predicts social influence" Current Biology 22(4), R123-R124.
- ↑ Tanferna A.; López-Jiménez L.; Blas J.; Hiraldo F.; Sergio F. (2012). "How Expert Advice Influences Decision Making". PLoS ONE. 7: 11.
- ↑ Astolfi, L., Cincotti, F., Mattia, D., De Vico Fallani, F., Salinari, S., Vecchiato, G., ... & Babiloni, F. (2010, August). Imaging the social brain: multi-subjects EEG recordings during the "Chicken's game". In Engineering in Medicine and Biology Society (EMBC), 2010 Annual International Conference of the IEEE (pp. 1734-1737). IEEE.
- ↑ Meyers C. A.; Berman S. A.; Scheibel R. S.; Hayman A. (1992). "Case report: acquired antisocial personality disorder associated with unilateral left orbital frontal lobe damage". Journal of psychiatry and neuroscience. 17 (3): 121.
- ↑ Kapogiannis D.; Barbey A. K.; Su M.; Krueger F.; Grafman J. (2009). "Neuroanatomical variability of religiosity". PLOS ONE. 4 (9): e7180. doi:10.1371/journal.pone.0007180.
- ↑ Nestor P. G.; Nakamura M.; Niznikiewicz M.; Thompson E.; Levitt J. J.; Choate V.; McCarley R. W. (2013). "In search of the functional neuroanatomy of sociality: MRI subdivisions of orbital frontal cortex and social cognition". Social cognitive and affective neuroscience. 8 (4): 460–467. doi:10.1093/scan/nss018.
- ↑ Spitzer M.; Fischbacher U.; Herrnberger B.; Grön G.; Fehr E. (2007). "The neural signature of social norm compliance". Neuron. 56 (1): 185–196. doi:10.1016/j.neuron.2007.09.011.
- ↑ Schwartz C. E.; Kunwar P. S.; Greve D. N.; Moran L. R.; Viner J. C.; Covino J. M.; Wallace S. R. (2010). "Structural differences in adult orbital and ventromedial prefrontal cortex predicted by infant temperament at 4 months of age". Archives of General Psychiatry. 67 (1): 78–84. doi:10.1001/archgenpsychiatry.2009.171.
- ↑ Stuss, D. T., Benson, D. F. (1986). The frontal lobes. New York: Raven Press.
- 1 2 3 4 5 6 Volkow N. D.; Ding Y. S.; Fowler J. S.; Wang G. J. (1996). "Cocaine addiction: hypothesis derived from imaging studies with PET". Journal of Addictive Diseases. 15: 55–71. doi:10.1300/j069v15n04_04.
- ↑ Volkow N. D.; Fowler J. S.; Wolf A. P.; Hitzemann R.; Dewey S.; Bendriem B.; Alpert R.; Hoff A. (1991). "Changes in brain glucose metabolism in cocaine dependence and withdrawal". American Journal of Psychiatry. 148: 621–626. doi:10.1176/ajp.148.5.621.
- ↑ Volkow N. D.; Hitzemann R.; Wang G. J.; Fowler J. S.; Wolf A. P.; Dewey S. L. (1992). "Long-term frontal brain metabolic changes in cocaine abusers". Synapse. 11: 184–190.
- ↑ Lingford-Hughes, A. R., Acton, P. D., Gacinovic, S., Suckling, J., Busatto, G. F., Boddington, S. J., Bullmore, E., Woodruff, P. W., Costa, D. C., Pilowsky, L. S., Ell, P. J. , Marshall, E. J., Kerwin, R. W. (1998).
- ↑ Koob G. F.; Bloom F. E. (1988). "Cellular and molecular mechanisms of drug dependence". Science. 242: 715–723. doi:10.1126/science.2903550.
- 1 2 Ray J. P.; Price J. L. (1993). "The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys". Journal of Comparative Neurology. 337: 1–31. doi:10.1002/cne.903370102.
- ↑ Pontieri F. E.; Tanda G.; Orzi F.; Chiara G. (1996). "Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs". Nature. 382: 255–257. doi:10.1038/382255a0.
- ↑ Haber S. N.; Kunishio K.; Mizobuchi M.; Lynd-Balta E. (1995). "The orbital and medial prefrontal circuit through the primate basal ganglia". The Journal of Neuroscience. 15: 4851–4867.
- ↑ Oades R. D.; Halliday G. M. (1987). "Ventral tegmental (A10) system: neurobiology: anatomy and connectivity". Brain Research. 434: 117–65.
- ↑ Carmichael S. T.; Price J. L. (1995). "Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys". The Journal of Comparative Neurology. 363: 615–641. doi:10.1002/cne.903630408.
- ↑ Thorpe S. J.; Rolls E. T.; Madison S. (1983). "The orbitofrontal cortex: neuronal activity in the behaving monkey". Experimental Brain Research. 49: 93–115. doi:10.1007/bf00235545.
- ↑ Johnson T. N. (1971). "Topographic projections in the globus pallidus and the substantia nigra of selectively placed lesions in the precommissural caudate nucleus and putamen in the monkey". Experimental Neurology. 33: 584–596. doi:10.1016/0014-4886(71)90129-4.
- ↑ Brown E. E.; Robertson G. S.; Fibiger H. C. (1992). "Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures". Neuroscience. 12: 4112–4121.
- ↑ Corso T. D.; Mostafa H. M.; Collins M.A.; Neafsey E.J. (1998). "Brain neuronal degeneration caused by episodic alcohol intoxication in rats: effects of nimodipine, 6,7-dinitro-quinoxaline-2,3-dione, and MK-801". Alcoholism: Clinical and Experimental Research. 22: 217–224. doi:10.1111/j.1530-0277.1998.tb03641.x.
- ↑ J. Wilson; M. Jenkinson; I. E. T. de Araujo; Morten L. Kringelbach; E. T. Rolls & Peter Jezzard (October 2002). "Fast, fully automated global and local magnetic field optimization for fMRI of the human brain". NeuroImage. 17 (2): 967–976. doi:10.1016/S1053-8119(02)91172-9. PMID 12377170.
- ↑ Kringelbach, M. L. & Rolls, E. T. (2004). "The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology". Progress in Neurobiology. 72 (5): 341–372. doi:10.1016/j.pneurobio.2004.03.006. PMID 15157726.
- ↑ Jay A. Gottfrieda & David H. Zald (2005). "On the scent of human olfactory orbitofrontal cortex: Meta-analysis and comparison to non-human primates". Brain Research Reviews. 50: 287–304. doi:10.1016/j.brainresrev.2005.08.004.
- ↑ Rolls E. T.; Hornak J.; Wade D.; McGrath J. (1994). "Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage". J Neurol Neurosurg Psychiatry. 57: 1518–1524. doi:10.1136/jnnp.57.12.1518.
- ↑ Bechara A.; Damasio A. R.; Damasio H.; Anderson S.W. (1994). "Insensitivity to future consequences following damage to human prefrontal cortex". Cognition. 50: 7–15. doi:10.1016/0010-0277(94)90018-3.
- ↑ Stone V.E.; Baron-Cohen S.; Knight R. T. (1998a). "Frontal Lobe Contributions to Theory of Mind". Journal of Medical Investigation. 10: 640–656. doi:10.1162/089892998562942.
- ↑ Snowden J. S.; Bathgate D.; Varma A.; Blackshaw A.; Gibbons Z. C.; Neary D. (2001). "Distinct behavioural profiles in frontotemporal dementia and semantic dementia". J Neurol Neurosurg Psychiatry. 70: 323–332. doi:10.1136/jnnp.70.3.323.
External links
| Wikimedia Commons has media related to Orbitofrontal cortex. |
- Cerebral Cortex special issue on orbitofrontal cortex
- Camille et al. (2004) The Involvement of the Orbitofrontal Cortex in the Experience of Regret