Ventral tegmental area
| Ventral tegmental area | |
|---|---|
 Transverse section of mid-brain at level of superior colliculi. (Tegmentum labeled at center right.) | |
| Details | |
| Identifiers | |
| Latin | Area tegmentalis ventralis |
| NeuroNames | hier-512 |
| NeuroLex ID | Ventral tegmentum |
The ventral tegmental area (VTA), (tegmentum is Latin for covering), also known as the ventral tegmental area of Tsai,[1] or simply ventral tegmentum, is a group of neurons located close to the midline on the floor of the midbrain. The VTA is the origin of the dopaminergic cell bodies of the mesocorticolimbic dopamine system and is widely implicated in the drug and natural reward circuitry of the brain. It is important in cognition, motivation, orgasm,[2] drug addiction, intense emotions relating to love, and several psychiatric disorders. Neurons in the VTA project to numerous areas of the brain, from the prefrontal cortex to the caudal brainstem and several regions in between.
Anatomy
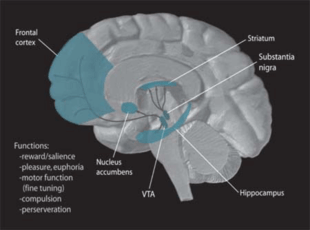
Neurobiologists have often had great difficulty distinguishing the VTA in humans and other primate brains from the substantia nigra (SN) and surrounding nuclei. Originally, the ventral tegmentum was designated as a ‘nucleus,’ but over time ‘area’ became the more appropriate term used because of the heterogeneous cytoarchitectonic features of the region and the lack of clear borders that separate it from adjacent regions. Because of the selective limbic-related afferents to the VTA, the cells of the VTA are given the designation A10 to differentiate them from surrounding cells.
Location
The ventral tegmental area is in the midbrain between several other major areas, some of which are described here. The mammillary bodies and the posterior hypothalamus, both included in the diencephalon, extend rostrally from the VTA. The red nucleus is situated laterally and oculomotor fibers are situated ventromedially to the VTA. The pons and the hindbrain lie caudally to the VTA. Finally, the substantia nigra is located laterally to the VTA.
Structure
In 1987, Oades identified four primary nuclei in the VTA A10 group of cells: the nucleus paranigralis (Npn), the nucleus parabrachialis pigmentosus (Npbp), the nucleus interfascicularis (Nif), and the nucleus linearis (Nln) caudalis and rostralis. Presently, scientists divide the VTA up into four similar zones that are called the paranigral nucleus (PN), the parabrachial pigmented area (PBP), the parafasciculus retroflexus area (PFR), and the rostromedial tegmental nucleus (RMTg), which approximately adhere to the previous divisions. Some definitions of the VTA also include the midline nuclei (i.e. the interfascicular nucleus, rostral linear nucleus, and central linear nucleus).
The PN and PBP are rich in dopaminergic cells, whereas the other two regions have low densities of these neurons. The PFR and RMTg contain a low density of tyrosine hydroxylase (TH)-positive cell bodies that are small in size and lightly stain; the RMTg is composed mostly of GABAergic cells. On the other hand, the PN and PBP consist mainly of medium to large sized TH-positive cell bodies that stain moderately.
Function
As stated above, the VTA, in particular the VTA dopamine neurons, serve several functions in the reward system, motivation, cognition, drug addiction, and may be the focus of several psychiatric disorders. It has also been shown to process various types of emotion output from the amygdala, where it may also play a role in avoidance and fear-conditioning. Electrophysiological recordings have demonstrated that VTA neurons respond to novel stimuli, unexpected rewards, and reward-predictive sensory cues. The firing pattern of these cells is consistent with the encoding of a reward expectancy error.
In 2006 MRI Studies by Helen Fisher and her research team found and documented various emotional states relating to intense love correlated with activity in the VTA, which may help explain obsessive behaviors of rejected partners since this is shared by the reward system. Nest sharing behavior is associated with increased V1aR expression in the VTA of newly paired zebra finches.[3] However, V1aR expression was not related to female directed song rates, which may indicate a selective role of vasotocin in the VTA on pair maintenance versus courtship behavior.[3]
Presence of gap junctions
The VTA has been shown to have a large network of GABAergic neurons that are interconnected via gap junctions. This network allows for electrical conduction, which is considerably faster than the chemical conduction of signals between synapses.
Development
Because they develop from common embryonic tissue and partly overlap in their projection fields, Dopaminergic cell groups lack clear anatomical boundaries. During the development of the mammalian brain, both substantia nigra (SN) and VTA neurons initially project to the dorsolateral and ventromedial striatum. However, at birth the SN dopaminergic neurons project exclusively into the dorsolateral striatum, and the VTA dopaminergic neurons project solely into the ventromedial striatum. This pruning of connections occurs through the elimination of the unnecessary collaterals.
Neural composition
The VTA, like the substantia nigra, is populated with melanin-pigmented dopaminergic neurons.[4] Recent studies have suggested that dopaminergic neurons comprise 50-60% of all neurons in the VTA,[5] which is contrary to previous evidence that noted 77% of neurons within the VTA to be dopaminergic.[6] In addition, there is a sizable population of GABAergic neurons in the rostromedial tegmental nucleus (RMTg), a functionally distinct brain structure.[7][8] These GABAergic neurons regulate the firing of their dopaminergic counterparts that send projections throughout the brain to, but not limited to, the following regions: the prefrontal cortex, the nucleus accumbens, and the locus coeruleus. The VTA also contains a small percentage of excitatory glutamatergic neurons.
Comparative anatomy
All studies since 1964 have emphasized the impressive general similarity between the VTA of all mammals from rodents to humans. These studies have focused their efforts on rats, rabbits, dogs, cats, opossum, non-human primates, and humans. There have been slight differences noted, such as changes in the dorsal extent of the A10 cells. To be specific, the dorsal peak of A10 cells is more extensive in primates when compared to other mammals. Furthermore, the number of dopaminergic cells in the VTA increases with phylogenetic progression; for instance, the VTA of the mouse contains approximately 25,000 neurons, while the VTA of a 33-year-old man contains around 450,000 cell bodies.
Outputs
The two primary efferent fiber projections of the VTA are the mesocortical and the mesolimbic pathways, which correspond to the prefrontal cortex and nucleus accumbens respectively.[9][10] The full set of projections, all of which utilize dopamine as their primary neurotransmitter, is listed below.[9]
- Ventral tegmental area (VTA) projections[9]
- VTA → Amygdala
- VTA → Cingulate gyrus
- VTA → Hippocampus
- VTA → Nucleus accumbens
- VTA → Olfactory bulb
- VTA → Prefrontal cortex
Inputs
Almost all areas receiving projections from the VTA project back to it. Thus, the ventral tegmentum is reciprocally connected with a wide range of structures throughout the brain suggesting that it has a role in the control of function in the phylogenetically new and highly developed neocortex, as well as that of the phylogenetically older limbic areas.
There are excitatory glutamatergic afferents that arise from most structures that project into the VTA. These glutamatergic afferents play a key role in regulating VTA cell firing. When the glutamatergic neurons are activated, the firing rates of the dopamine neurons increase in the VTA and induce burst firing. Studies have shown that these glutamatergic actions in the VTA are critical to the effects of drugs of abuse. In contrast, the tVTA (also known as the RMTg) projects to the VTA with GABAergic afferents, functioning as a "master brake" for the VTA dopamine pathways.[7][8]
Subpallidal afferents into the VTA are mainly GABAergic and, thus, inhibitory. There is a substantial pathway from the subpallidal area to the VTA. When this pathway is disinhibited, an increase in the dopamine release in the mesolimbic pathway amplifies locomotor activity.
Limbic loop
The “limbic loop” is very similar to the direct pathway motor loop of the basal ganglia. In both systems, there are major excitatory inputs from the cortex to the striatum (accumbens nucleus), the midbrain project neuromodulatory dopamine neurons to the striatum, the striatum makes internuclear connections to the pallidum, and the pallidum has outputs to the thalamus, which projects to the cortex, thus completing the loop. The limbic loop is distinguished from the motor loop by the source and nature of the cortical input, the division of the striatum and pallidum that process the input, the source of the dopaminergic neurons form the midbrain, and the thalamic target of the pallidal output.
CA3 loop
Linking context to reward is important for reward seeking. In 2011, a group of researchers documented a VTA-CA3 loop that uses the lateral septum as an intermediary. They used a pseudo-rabies virus (PRV) as a transsynaptic tracer, and injected it into the VTA. They found that unilateral injection into the VTA resulted in bilateral PRV labeling in CA3 beginning 48 hours after injection. Lesions of the caudodorsal lateral septum (cd-LS) prior to VTA PRV injection resulted in significantly less PRV labeled neurons in CA3. Theta wave stimulation of CA3 resulted in increased firing rates for dopamine cells in the VTA, and decreased firing rates for GABA neurons in the VTA. The identity of VTA neurons was confirmed by neurobiotin™ labeling of the recording neuron, and then histological staining for tyrosine hydroxylase (TH). Temporary inactivation of CA3 via GABA agonists prevented context induced reinstatement of lever pressing for intravenous cocaine.[11]
The authors propose a functional circuit loop where activation of glutamatergic cells in CA3 causes activation of GABAergic cells in cd-LS, which inhibits GABA interneurons in the VTA, releasing the dopamine cells from the tonic inhibition, and leading to an increased firing rate for the dopamine cells.[11]
Reward system
The dopamine reward circuitry in the human brain involves two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. First, the posteromedial VTA and central linear raphe cells selectively project to the ventromedial striatum, which includes the medial olfactory tubercle and the medial NAC shell. Second, the lateral VTA projects largely to the ventrolateral striatum, which includes the NAC core, the medial NAC shell, and the lateral olfactory tubercle. These pathways are called the meso-ventromedial and the meso-ventrolateral striatal dopamine systems, respectively. The medial projection system is important in the regulation of arousal characterized by affect and drive and plays a different role in goal-directed behavior than the lateral projection system. Unlike the lateral part, the medial one is activated not by rewarding but by noxious stimuli. [12] [13] Therefore, the NAC shell and the posterior VTA are the primary areas involved in the reward system .
Normally, the dopaminergic neurons are only phasically active. When they are excited, they fire a barrage of action potentials and dopamine is released in the NAC. The medium spiny neurons of the NAC are much more responsive to this increase in dopamine if there is coincident excitatory input from the telencephalic structures such as the amygdala and orbital-medial prefrontal cortex. The activated striatal neurons (NAC neurons) then project to the ventral pallidum, where they inhibit the inhibitory GABA neurons. This inhibition in the pallidum disinhibits the thalamic target of the limbic loop, which is the mediodorsal nucleus. The thalamus then innervates the cortical division of the limbic forebrain. This final connection is reinforced by activity in direct cortical projections from the dopaminergic neurons of the VTA.
Clinical relevance
Disorders
The dopaminergic neurons of the substantia nigra and the ventral tegmental area of the midbrain project to the dorsolateral caudate/putamen and to the ventromedially located nucleus accumbens, respectively, establishing the mesostriatal and the mesolimbic pathways. The close proximity of these two pathways causes them to be grouped together under dopaminergic projections. Several disorders result from the disruption of these two pathways: schizophrenia, Parkinson's disease, and attention deficit hyperactivity disorder (ADHD). Current research is examining the subtle difference between the neurons that are involved in these conditions and trying to find a way to selectively treat a specific dopamine projection.
Drug addiction
The nucleus accumbens and the ventral tegmentum are the primary sites where addictive drugs act. The following are commonly considered to be addictive: heroin, cocaine, alcohol, opiates, nicotine, amphetamine, and their synthetic analogs. These drugs alter the neuromodulatory influence of dopamine on the processing of reinforcement signals by prolonging the action of dopamine in the nucleus accumbens or by stimulating the activation of neurons there and also in the VTA. The most common drugs of abuse stimulate the release of dopamine, which creates both their rewarding and the psychomotor effects. Compulsive drug-taking behaviors are a result of the permanent functional changes in the mesolimbic dopamine system arising from repetitive dopamine stimulation. Molecular and cellular adaptations are responsible for a sensitized dopamine activity in the VTA and along the mesolimbic dopamine projection in response to drug abuse. In the VTA of addicted individuals, the activity of the dopamine-synthesizing enzyme tyrosine hydroxylase increases, as does the ability of these neurons to respond to excitatory inputs. The latter effect is secondary to increases in the activity of the transcription factor CREB and the up regulation of GluR1, an important subunit of AMPA receptors for glutamate. These alterations in neural processing could account for the waning influence of adaptive emotional signals in the operation of decision making faculties as drug-seeking and drug-taking behaviors become habitual and compulsive.
Experiments in rats have shown that they learn to press a lever for the administration of stimulant drugs into the posterior VTA more readily than into the anterior VTA. Other studies have shown that microinjections of dopaminergic drugs into the nucleus accumbens shell, increase locomotor activity and exploratory behaviors, conditioned approach responses, and anticipatory sexual behaviors.
The withdrawal phenomenon occurs because the deficit in reward functioning initiates a distress cycle wherein the drugs become necessary to restore the normal homeostatic state. Recent research has shown that even after the final stages of withdrawal have been passed, drug-seeking behavior can be reinstated if exposed to the drug or drug-related stimuli.
See also
References
- ↑ Phillipson, O. T. (1979). "Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: A horseradish peroxidase study in the rat". The Journal of Comparative Neurology. 187 (1): 117–143. doi:10.1002/cne.901870108. PMID 489776.
- ↑ Holstege, G.; Georgiadis, J. R.; Paans, A. M.; Meiners, L. C.; Van Der Graaf, F. H.; Reinders, A. A. (2003). "Brain activation during human male ejaculation". The Journal of neuroscience : the official journal of the Society for Neuroscience. 23 (27): 9185–9193. PMID 14534252.
- 1 2 Tomaszycki, ML; Richardson, KK; Mann, KJ (October 2016). "Sex and pairing status explain variations in the activation of nonapeptide receptors in song and motivation regions.". Behavioral neuroscience. 130 (5): 479–489. PMID 27504854.
- ↑ http://cogprints.org/1390/2/vta-rev-87.pdf
- ↑ Margolis EB, Lock H, Hjelmstad GO, Fields HL. 2006b. The ventral tegmental area revisited: Is there an electrophysiological marker for dopaminergic neurons? J. Physiol. 577(3) 907–24
- ↑ Johnson, SW; North, RA (May 1992). "Two types of neurone in the rat ventral tegmental area and their synaptic inputs.". The Journal of Physiology. 450: 455–68. doi:10.1113/jphysiol.1992.sp019136. PMC 1176131
 . PMID 1331427.
. PMID 1331427. - 1 2 Bourdy R, Barrot M (November 2012). "A new control center for dopaminergic systems: pulling the VTA by the tail". Trends Neurosci. 35 (11): 681–690. doi:10.1016/j.tins.2012.06.007. PMID 22824232.
In light of the crucial role of the tVTA in the opiate control of dopamine activity ...
In the context of addiction, the tVTA is a target for psychostimulant-induced plasticity [1,6,23] and is also essential for morphine action on dopamine neurons [19]. This latter finding suggests that the classical disinhibition model may need to be revisited in light of the GABAergic control that the tVTA exerts on dopamine systems. ...
The tVTA is rich in inhibitory GABA neurons expressing μ-opioid receptors and sends extensive projections toward midbrain dopamine cells. It is proposed as a major brake for dopamine systems. ...
The tVTA was initially described in rats as a bilateral cluster of GABA neurons within the posterior VTA, dorsolateral to the interpeduncular nucleus, and expressing FosB/ΔFosB after psychostimulant administration [1]. However, the Fos staining showed that this group of cells extends caudally beyond the defined borders of the VTA [1], shifting dorsally to become embedded within the superior cerebellar peduncle [2]. Around the same time as the tVTA was described, a region caudal to the rat VTA and lateral to the median raphe was proposed to influence passive aversive responses [24]. This region belongs to the reticular formation and was later designated as RMTg [3]. The RMTg extends rostrally, shifting ventrally to become embedded within the posterior VTA. A similar region has also been observed in primates [18] and in mice [25]. There is now agreement that the tVTA and RMTg are two faces of the same structure. - 1 2 Barrot M, Sesack SR, Georges F, Pistis M, Hong S, Jhou TC (October 2012). "Braking dopamine systems: a new GABA master structure for mesolimbic and nigrostriatal functions". J. Neurosci. 32 (41): 14094–14101. doi:10.1523/JNEUROSCI.3370-12.2012. PMC 3513755
 . PMID 23055478.
. PMID 23055478. The tVTA/RMTg sends dense GABA projections to VTA and substantia nigra neurons. ...
Indeed, tVTA/RMTg cells express high levels of mu-opioid receptors (Jhou et al., 2009a, 2012; Jalabert et al., 2011), and in vivo, ex vivo and optogenetic electrophysiological approaches demonstrated that morphine excites dopamine neurons by targeting receptors localized to tVTA/RMTg cell bodies as well as its terminals within the VTA (Jalabert et al., 2011; Lecca et al., 2011; Matsui and Williams, 2011; Lecca et al., 2012). ... Recent research on the tVTA/RMTg started from observations related to psychostimulant induction of FosB/ΔFosB (Perrotti et al., 2005) and to the control of aversive responses (Jhou, 2005). The rat tVTA/RMTg showed a neuroanatomically delimited increase in the expression of Fos-related proteins following exposure to psychostimulants (Scammel et al., 2000; Perrotti et al., 2005; Geisler et al., 2008; Jhou et al., 2009a; Kaufling et al., 2009, 2010a, 2010b; Rottlant et al., 2010; Zahm et al., 2010; Cornish et al., 2012). This induction was observed with both acute and chronic exposure to psychostimulants, and with both self-administration and non-contingent administration. There is a strong selectivity of this molecular response, as the Fos-related induction was never observed with non-psychostimulant drugs (Perrotti et al., 2005; Kaufling et al., 2010b). - 1 2 3 Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin". In Sydor A, Brown RY. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 147–148, 154–157. ISBN 9780071481274.
Neurons from the SNc densely innervate the dorsal striatum where they play a critical role in the learning and execution of motor programs. Neurons from the VTA innervate the ventral striatum (nucleus accumbens), olfactory bulb, amygdala, hippocampus, orbital and medial prefrontal cortex, and cingulate cortex. VTA DA neurons play a critical role in motivation, reward-related behavior, attention, and multiple forms of memory. ... Thus, acting in diverse terminal fields, dopamine confers motivational salience ("wanting") on the reward itself or associated cues (nucleus accumbens shell region), updates the value placed on different goals in light of this new experience (orbital prefrontal cortex), helps consolidate multiple forms of memory (amygdala and hippocampus), and encodes new motor programs that will facilitate obtaining this reward in the future (nucleus accumbens core region and dorsal striatum). ... DA has multiple actions in the prefrontal cortex. It promotes the "cognitive control" of behavior: the selection and successful monitoring of behavior to facilitate attainment of chosen goals. Aspects of cognitive control in which DA plays a role include working memory, the ability to hold information "on line" in order to guide actions, suppression of prepotent behaviors that compete with goal-directed actions, and control of attention and thus the ability to overcome distractions. ... Noradrenergic projections from the LC thus interact with dopaminergic projections from the VTA to regulate cognitive control. ...
- ↑ Nechifor M (March 2008). "Magnesium in drug dependences". Magnes Res. 21 (1): 5–15. PMID 18557129.
- 1 2 Luo, Alice; Tahsili-Fahadan, P.; Wise, R. A.; Lupica, C. R.; Aston-Jones, G. (July 2011). "Linking Context with Reward: A Functional Circuit From Hippocampal CA3 to Ventral Tegmental Area". Science. 333 (6040): 353–356. doi:10.1126/science.1204622. PMC 3150711
 . PMID 21764750.
. PMID 21764750. - ↑ Ikemoto, S (2007). "Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex". Brain Res Rev. 56: 27–78. doi:10.1016/j.brainresrev.2007.05.004. PMC 2134972
 . PMID 17574681.
. PMID 17574681. - ↑ Brischoux, F; Chakraborty, S; Brierley, DI; Ungless, MA (2009). "Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli". Proceedings of the National Academy of Sciences of the United States of America. 106 (12): 4894–9. doi:10.1073/pnas.0811507106. PMC 2660746
 . PMID 19261850.
. PMID 19261850.
Further reading
- Alcaro, A; Huber, R; Panksepp, J (Dec 2007). "Behavioral functions of the mesolimbic dopaminergic system: An affective neuroethological perspective". Brain Research Reviews. 56 (2): 283–321. doi:10.1016/j.brainresrev.2007.07.014.
- Geisler, S; Derst, C; Veh, RW; Zahm, DS (May 2007). "Glutamatergic afferents of the ventral tegmental area in the rat". Journal of Neuroscience. 27 (21): 5730–43. doi:10.1523/jneurosci.0012-07.2007.
- Hikosaka, O; Bromberg-Martin, E; Hong, S; Matsumoto, M (Apr 2008). "New insights on the subcortical representation of reward". Current Opinion in Neurobiology. 18 (2): 203–8. doi:10.1016/j.conb.2008.07.002.
- Hu, ZL; Cooper, M; Crockett, DP; Zhou, RP (Aug 2004). "Differentiation of the midbrain dopaminergic pathways during mouse development". Journal of Comparative Neurology. 476 (3): 301–11. doi:10.1002/cne.20230.
- Ikemoto, S (Nov 2007). "Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex". Brain Research Reviews. 56 (1): 27–78. doi:10.1016/j.brainresrev.2007.05.004. PMC 2134972
 . PMID 17574681.
. PMID 17574681. - Lammel, S; Hetzel, A; Haeckel, O; Jones, I; Liss, B; Roeper, J (Mar 2008). "Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system". Neuron. 57 (5): 760–73. doi:10.1016/j.neuron.2008.01.022.
- Lu, XY; Ghasemzadeh, MB; Kalivas, PW (Feb 1998). "Expression of D-1 receptor, D-2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens". Neuroscience. 82 (3): 767–80. doi:10.1016/s0306-4522(97)00327-8.
- Margolis, EB; Lock, H; Hjelmstad, GO; Fields, HL (Dec 2006). "The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons?". Journal of Physiology. 577 (3): 907–24. doi:10.1113/jphysiol.2006.117069.
- Oades, RD; Halliday, GM (May 1987). "VENTRAL TEGMENTAL (A10) SYSTEM - NEUROBIOLOGY .1. ANATOMY AND CONNECTIVITY". Brain Research Reviews. 12 (2): 117–65. doi:10.1016/0165-0173(87)90011-7.
- Olson, VG; Nestler, EJ (Feb 2007). "Topographical organization of GABAergic neurons within the ventral tegmental area of the rat". Synapse. 61 (2): 87–95. doi:10.1002/syn.20345.
- Sziráki I, Sershen H, Hashim A, Lajtha A (2002). "Receptors in the ventral tegmental area mediating nicotine-induced dopamine release in the nucleus accumbens". Neurochem. Res. 27 (3): 253–61. PMID 11958525.
- van Furth WR, van Ree JM (1996). "Sexual motivation: involvement of endogenous opioids in the ventral tegmental area". Brain Res. 729 (1): 20–8. doi:10.1016/s0006-8993(96)00225-9. PMID 8874873.
- Wu, M; Hrycyshyn, AW; Brudzynski, SM (Nov 1996). "Subpallidal outputs to the nucleus accumbens and the ventral tegmental area: Anatomical and electrophysiological studies". Brain Research. 740 (1-2): 151–61. doi:10.1016/s0006-8993(96)00859-1.
| Wikimedia Commons has media related to Ventral tegmental area. |