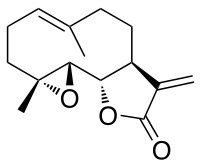
Parthenolide
 | |
| Names | |
|---|---|
| IUPAC name
(3aS,9aR,10aS,10bS,E)-6,9a-dimethyl-3-methylene-3a,4,5,8,9,9a,10a,10b-octahydrooxireno[2',3':9,10]cyclodeca[1,2-b]furan-2(3H)-one | |
| Identifiers | |
| 20554-84-1 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL540445 |
| ChemSpider | 20126246 |
| ECHA InfoCard | 100.220.558 |
| PubChem | 5420805 |
| RTECS number | LY4220000 |
| UNII | 2RDB26I5ZB |
| |
| |
| Properties | |
| C15H20O3 | |
| Molar mass | 248.32 g·mol−1 |
| Melting point | 113 to 115 °C (235 to 239 °F; 386 to 388 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Parthenolide is a sesquiterpene lactone of the germacranolide class which occurs naturally in the plant feverfew (Tanacetum parthenium), after which it is named. It is found in highest concentration in the flowers and fruit. Feverfew is used in herbalism and is purportedly useful for a variety of aliments. Many vendors of feverfew remedies specify the content of parthenolide in their products, because it is believed to be the primary chemical constituent responsible for biological activity.[1]
Lack of solubility in water and bioavailability limits the potential of parthenolide as a drug. Drug researchers are trying to develop synthetic analogs instead that will be absorbed to a more useful extent.[2] It also inhibits HDAC1 protein without affecting other class I/II HDACs, which leads to sustained DNA damage response in certain cells (required for apoptosis).[3]
In vitro biological activities
Parthenolide has a variety of reported in vitro biological activities, including:
- Modulation of the NF-κB-mediated inflammatory responses in experimental atherosclerosis.[4]
- intraneural or systemic application promotes axon regeneration in the peripheral nervous system [5]
- Inducing apoptosis in acute myelogenous leukemia (AML) cells, leaving normal bone marrow cells relatively unscathed. Moreover, the compound may get at the root of the disease because it also kills stem cells that give rise to AML.[6] Parthenolide is under consideration as a potential cancer drug in combination with sulindac.
- Activity against a parasite Leishmania amazonensis.[7]
- Microtubule-interfering activity.[8]
- Anti-inflammatory and anti-hyperalgesic effects.[9]
- Blocking lipopolysaccharide-induced osteolysis through the suppression of NF-κB activity.[10]
- inducing apoptosis and production of reactive oxygen species in high-risk pre-B leukemia cells.[11]
- Parthenolide induces MITF M isoform downregulation and senescence in MITF-Mhigh melanoma cells[12]
Parthenolide has been found to act as an agonist of the adiponectin receptor 2 (AdipoR2).[13]
References
- ↑ Parthenolide from Fermentek
- ↑ Will Boggs. "Orally Bioavailable Parthenolide Analog Eradicates Leukemia Stem Cells". Reuters Health.
- ↑ Rajendran, P; Ho, E; Williams, DE; Dashwood, RH (2011). "Dietary phytochemicals, HDAC inhibition, and DNA damage/repair defects in cancer cells". Clin Epigenetics. 3: 4. doi:10.1186/1868-7083-3-4. PMC 3255482
 . PMID 22247744.
. PMID 22247744. - ↑ López-Franco, O; Hernández-Vargas, P; Ortiz-Muñoz, G; Sanjuán, G; Suzuki, Y; Ortega, L; Blanco, J; Egido, J; Gómez-Guerrero, C (2006). "Parthenolide modulates the NF-kappaB-mediated inflammatory responses in experimental atherosclerosis". Arteriosclerosis, thrombosis, and vascular biology. 26 (8): 1864–70. doi:10.1161/01.ATV.0000229659.94020.53. PMID 16741149.
- ↑ Gobrecht, P.; Andreadaki, A.; Diekmann, H.; Heskamp, A.; Leibinger, M.; Fischer, D. (6 April 2016). "Promotion of Functional Nerve Regeneration by Inhibition of Microtubule Detyrosination". Journal of Neuroscience. 36 (14): 3890–3902. doi:10.1523/JNEUROSCI.4486-15.2016.
- ↑ Guzman, ML; Rossi, RM; Karnischky, L; Li, X; Peterson, DR; Howard, DS; Jordan, CT (2005). "The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells". Blood. 105 (11): 4163–9. doi:10.1182/blood-2004-10-4135. PMC 1895029
 . PMID 15687234.
. PMID 15687234. - ↑ Tiuman, TS; Ueda-Nakamura, T; Garcia Cortez, DA; Dias Filho, BP; Morgado-Díaz, JA; De Souza, W; Nakamura, CV (2005). "Antileishmanial Activity of Parthenolide, a Sesquiterpene Lactone Isolated from Tanacetum parthenium". Antimicrobial Agents and Chemotherapy. 49 (1): 176–82. doi:10.1128/AAC.49.11.176-182.2005. PMC 538891
 . PMID 15616293.
. PMID 15616293. - ↑ Miglietta, A; Bozzo, F; Gabriel, L; Bocca, C (2004). "Microtubule-interfering activity of parthenolide". Chemico-biological interactions. 149 (2–3): 165–73. doi:10.1016/j.cbi.2004.07.005. PMID 15501437.
- ↑ Feltenstein, MW; Schühly, W; Warnick, JE; Fischer, NH; Sufka, KJ (2004). "Anti-inflammatory and anti-hyperalgesic effects of sesquiterpene lactones from Magnolia and Bear's foot". Pharmacology, Biochemistry, and Behavior. 79 (2): 299–302. doi:10.1016/j.pbb.2004.08.008. PMID 15501305.
- ↑ Yip, KH; Zheng, MH; Feng, HT; Steer, JH; Joyce, DA; Xu, J (2004). "Sesquiterpene lactone parthenolide blocks lipopolysaccharide-induced osteolysis through the suppression of NF-kappaB activity". Journal of Bone and Mineral Research. 19 (11): 1905–16. doi:10.1359/JBMR.040919. PMID 15476591.
- ↑ Zunino, SJ; Ducore, JM; Storms, DH (2007). "Parthenolide induces significant apoptosis and production of reactive oxygen species in high-risk pre-B leukemia cells". Cancer Letters. 254 (1): 119–27. doi:10.1016/j.canlet.2007.03.002. PMID 17470383.
- ↑ Hartman, M. L., Talar, B., Sztiller-Sikorska, M., Nejc, D., & Czyz, M. (2016). Parthenolide induces MITF-M downregulation and senescence in patient-derived MITF-Mhigh melanoma cell populations. Oncotarget, 7(8), 9026-9040. doi:10.18632/oncotarget.7030
- ↑ Sun Y, Zang Z, Zhong L, Wu M, Su Q, Gao X, Zan W, Lin D, Zhao Y, Zhang Z (2013). "Identification of adiponectin receptor agonist utilizing a fluorescence polarization based high throughput assay". PLoS ONE. 8 (5): e63354. doi:10.1371/journal.pone.0063354. PMC 3653934
 . PMID 23691032.
. PMID 23691032.
External links
- Guzman, ML; Jordan, CT (2005). "Feverfew: weeding out the root of leukaemia". Expert opinion on biological therapy. 5 (9): 1147–52. doi:10.1517/14712598.5.9.1147. PMID 16120045.
- Headache herb may boost cancer survival, Indiana University Research and Creative Activity, Fall 2006
- Gobrecht et al (2016): Promotion of Functional Nerve Regeneration by Inhibition of Microtubule Detyrosination.[1]
- ↑ Gobrecht, P.; Andreadaki, A.; Diekmann, H.; Heskamp, A.; Leibinger, M.; Fischer, D. (6 April 2016). "Promotion of Functional Nerve Regeneration by Inhibition of Microtubule Detyrosination". Journal of Neuroscience. 36 (14): 3890–3902. doi:10.1523/JNEUROSCI.4486-15.2016.