Lisinopril
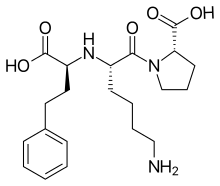 | |
 Chemical structure of lisinopril | |
| Clinical data | |
|---|---|
| Pronunciation | /laɪˈsɪnəprɪl/, ly-SIN-ə-pril |
| Trade names | Prinivil, Zestril, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692051 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | C09AA03 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | approx. 25%, but wide range between individuals (6 to 60%) |
| Protein binding | 0 |
| Metabolism | None |
| Biological half-life | 12 hours |
| Excretion | Eliminated unchanged in urine |
| Identifiers | |
| |
| Synonyms | (2S)-1-[(2S)-6-amino-2-{[(1S)-1-carboxy-3-phenylpropyl]amino}hexanoyl]pyrrolidine-2-carboxylic acid |
| CAS Number | 83915-83-7 |
| PubChem (CID) | 5362119 |
| IUPHAR/BPS | 6360 |
| DrugBank |
APRD00560 |
| ChemSpider |
4514933 |
| UNII |
7Q3P4BS2FD |
| KEGG |
D00362 |
| ChEBI |
CHEBI:43755 |
| ChEMBL |
CHEMBL1237 |
| PDB ligand ID | LPR (PDBe, RCSB PDB) |
| ECHA InfoCard | 100.155.382 |
| Chemical and physical data | |
| Formula | C21H31N3O5 |
| Molar mass | 405.488 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Lisinopril is a drug of the angiotensin-converting enzyme (ACE) inhibitor class used primarily in treatment of high blood pressure, heart failure, and after heart attacks. It is also used for preventing kidney and eye complications in people with diabetes. Its indications, contraindications, and side effects are as those for all ACE inhibitors.
Lisinopril was the third ACE inhibitor (after captopril and enalapril) and was introduced into therapy in the early 1990s.[3] A number of properties distinguish it from other ACE inhibitors: It is hydrophilic, has a long half-life and tissue penetration, and is not metabolized by the liver.
Medical uses
Lisinopril is typically used for the treatment of hypertension, congestive heart failure, acute myocardial infarction, and diabetic nephropathy.[4]
Contraindications
Treatment with lisinopril should be avoided for people who have a history of angioedema (hereditary or idiopathic) or who have diabetes and are taking aliskiren.[2]
Adverse effects
Side effects, incidence differs depending on which disease state the patient is being treated for.[2]
People taking lisinopril for the treatment of hypertension may experience the following side effects:
- Headache (3.8%)
- Dizziness (3.5%)
- Cough (2.5%)
- Difficulty swallowing or breathing (signs of angioedema), allergic reaction (anaphylaxis)
- Hyperkalemia (2.2% in adult clinical trials)
- Fatigue (1% or more)
- Diarrhea (1% or more)
- Some severe skin reactions have been reported rarely, including toxic epidermal necrolysis and Stevens-Johnson syndrome; causal relationship has not been established.
People taking lisinopril for the treatment of myocardial infarction may experience the following side effects:
- Hypotension (5.3%)
- Renal dysfunction (1.3%)
People taking lisinopril for the treatment of heart failure may experience the following side effects:
- Hypotension (3.8%)
- Dizziness (12% at low dose – 19% at high dose)
- Chest pain (2.1%)
- Fainting (5-7%)
- Hyperkalemia (3.5% at low dose – 6.4% at high dose)
- Difficulty swallowing or breathing (signs of angioedema), allergic reaction (anaphylaxis)
- Fatigue (1% or more)
- Diarrhea (1% or more)
- Some severe skin reactions have been reported rarely, including toxic epidermal necrolysis and Stevens-Johnson syndrome; causal relationship has not been established.
Special populations
Caution should be used in the following populations, as dose adjustments may be required.
Kidney problems
The dose must be adjusted in those with poor kidney function. Dose adjustments may be required when creatinine clearance is less than or equal to 30mL/min. Since lisinopril is removed by dialysis, dosing changes must also be considered for people on dialysis.[2]
Pregnancy and breastfeeding
Lisinopril has been assigned to pregnancy category D by the FDA. Animal and human data have revealed evidence of lethal harm to the embryo and teratogenicity associated with ACE inhibitors. No controlled data in human pregnancy are available. Birth defects have been associated with use of lisinopril in any trimester. However, there have been reports of death and increased toxicities to the fetus and newly born child with the use of lisinopril in the second and third trimesters. The label states, "When pregnancy is detected, discontinue Zestril as soon as possible." The manufacturer recommends mothers should not breastfeed while taking this medication because of the lack of safety data that currently exists.[2]
Pharmacology
Lisinopril is the lysine-analog of enalapril. Unlike other ACE inhibitors, it is not a prodrug and is excreted unchanged in the urine. In cases of overdosage, it can be removed from circulation by dialysis.[2]
Mechanism of action
Lisinopril is an ACE Inhibitor, meaning it blocks the actions of angiotensin converting enzyme (ACE) in the renin-angiotensin-aldosterone system (RAAS), keeping Angiotensin I from being converted to Angiotensin II. The inhibition of this system causes an overall decrease in blood pressure.[5]
Pharmacokinetics
The following is a summary of lisinopril's pharmacokinetics:[2][5]
Absorption
- Lisinopril has a poor bioavailability of 25% (Reduced to 16% in people with NYHA Class II-IV heart failure)
- Time to peak concentration is 7 hours
- Food has not been shown to affect absorption
Distribution
- Does not bind to proteins in the blood
- Lisinopril does not distribute as well in people with NYHA Class II–IV heart failure
Metabolism
- Lisinopril does not undergo any form of metabolism in the body
Elimination
- Lisinopril leaves the body completely unchanged in the urine
- The half-life of the drug is 12 hours. This is increased in people with kidney problems
History
Captopril, the first ACE inhibitor, is a functional and structural analog of a peptide derived from the venom of the jararaca, a Brazilian pit viper (Bothrops jararaca).[6] Enalapril is a derivative, designed by scientists at Merck to overcome the rash and bad taste caused by captopril.[7][8]:12–13 Enalapril is actually a prodrug; the active metabolite is enalaprilat.[9]
Scientists at Merck created lisinopril by systematically altering each structural unit of enalaprilat, substituting various amino acids. It turned out that adding lysine at one end of the drug had strong activity and was orally available; analogs of that compounds resulted in lisonopril, which takes its name from the discovery with lysine. Merck conducted clinical trials, and the drug was approved for hypertension in 1987 and congestive heart failure in 1993.[9]
The discovery posed a problem, since sales of enalapril were strong for Merck, and the company didn't want to diminish those sales. Merck ended up entering into an agreement with Zeneca under which Zeneca received the right to co-market lisinopril, and Merck received the exclusive rights to an earlier stage aldose reductase inhibitor drug candidate, a potential treatment for diabetes. Zeneca's marketing and brand name, "Zestril", turned out to be stronger than Merck's effort.[10] The drug became a blockbuster for Astrazeneca (formed in 1998), with annual sales in 1999 of $1.2B.[11]
The US patents expired in 2002.[11] Since then, lisinopril has been available under many brand names worldwide; some formulations include the diuretic hydrochlorothiazide.[1]
See also
References
- 1 2 Drugs.com International brands and formulations for lisinopril Page accessed April 23, 2016
- 1 2 3 4 5 6 7 Lisinopril label Revised: 08/2015
- ↑ Patchett A; et al. (1980). "A new class of angiotensin-converting enzyme inhibitors". Nature. 288 (5788): 280–3. doi:10.1038/288280a0. PMID 6253826.
- ↑ "Lisinopril". The American Society of Health-System Pharmacists. Retrieved 3 April 2011.
- 1 2 Katzung, Bertram (2012). Basic and Clinical Pharmacology. New York City, New York: McGraw Hill. pp. 175, 184–85. ISBN 978-0071764018.
- ↑ Patlak M (March 2004). "From viper's venom to drug design: treating hypertension". FASEB J. 18 (3): 421. doi:10.1096/fj.03-1398bkt. PMID 15003987.
- ↑ Jenny Bryan for The Pharmaceutical Journal, 17 Apr 2009 "From snake venom to ACE inhibitor—the discovery and rise of captopril"
- ↑ Jie Jack Li, "History of Drug Discovery". Chapter 1 in Drug Discovery: Practices, Processes, and Perspectives. Eds. Jie Jack Li, E. J. Corey. John Wiley & Sons, Apr 3, 2013 ISBN 9781118354469
- 1 2 Menard J and Patchett A. "Angiotensin-Converting Enzyme Inhibitors". pp. 14–76 in Drug Discovery and Design. Volume 56 of Advances in Protein Chemistry. Eds. Richards FM, Eisenberg DS, and Kim PS. Series Ed. Scolnick EM. Academic Press, 2001. ISBN 9780080493381. pp. 30–33
- ↑ David R. Glover. Vie D’or: Memoirs of a Pharmaceutical Physician. Troubador Publishing Ltd, 2016. ISBN 9781785894947. Merck Sharp and Dohme: lisinopril section
- 1 2 Express Scripts. Patent expirations
Further reading
- Fogari R, Zoppi A, Corradi L, Lazzari P, Mugellini A, Lusardi P (November 1998). "Comparative effects of lisinopril and losartan on insulin sensitivity in the treatment of non diabetic hypertensive patients". Br J Clin Pharmacol. 46 (5): 467–71. doi:10.1046/j.1365-2125.1998.00811.x. PMC 1873694
 . PMID 9833600.
. PMID 9833600. - Bussien JP, Waeber B, Nussberger J, Gomez HJ, Brunner HR (1985). "Once-daily lisinopril in hypertensive patients: Effect on blood pressure and the renin-angiotensin system". Curr Therap Res. 37: 342–51.