MN-25
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
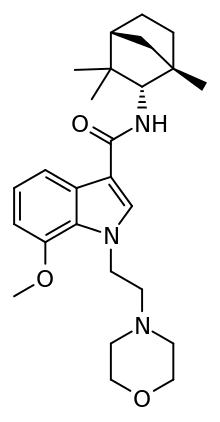
| Synonyms | N-[(S)-Fenchyl]-1-[2-(morpholin-4-yl)ethyl]-7-methoxyindole-3-carboxamide |
| CAS Number |
501926-82-5 2-methyl derivative: 501927-29-3 |
| PubChem (CID) | 71308243 |
| ChemSpider |
26286811 |
| UNII |
8WXU5YRE25 |
| ChEMBL | CHEMBL3234671 |
| Chemical and physical data | |
| Formula | C26H37N3O3 |
| Molar mass | 439.59 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb,[1] that acts as a reasonably selective agonist of peripheral cannabinoid receptors.[2] It has moderate affinity for CB2 receptors with a Ki of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a Ki of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a Ki of 8 nM at CB1 and 29 nM at CB2,[3][4] which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098).[5][6]

Chemically, it is closely related to another indole-3-carboxamide synthetic cannabinoid, Org 28611, but with a different cycloalkyl substitution on the carboxamide, and the cyclohexylmethyl group replaced by morpholinylethyl, as in JWH-200 or A-796,260. Early compounds such as these have subsequently led to the development of a large number of related indole-3-carboxamide cannabinoid ligands.[7][8][9][10]
See also
References
- ↑ CANNABINOID RECEPTOR MODULATORS, THEIR PROCESSES OF PREPARATION, AND USE OF CANNABINOID RECEPTOR MODULATORS IN TREATING RESPIRATORY AND NON-RESPIRATORY DISEASES. WO 2001/58869
- ↑ Rulin Zhao, et al. Improved procedure for the preparation of 7-methoxy-2-methyl-1-(2-morpholinoethyl)-1H-indole-3-carboxylic acid, key intermediate in the synthesis of novel 3-amidoindole and indolopyridone cannabinoid ligands. ARKIVOC 2010 (vi):89-95.
- ↑ Hynes, J.; et al. (2002). "C-3 Amido-Indole cannabinoid receptor modulators". Bioorganic & Medicinal Chemistry Letters. 12 (17): 2399–402. doi:10.1016/S0960-894X(02)00466-3. PMID 12161142.
- ↑ Wrobleski, Stephen T.; et al. (2003). "Rational Design and Synthesis of an Orally Active Indolopyridone as a Novel Conformationally Constrained Cannabinoid Ligand Possessing Antiinflammatory Properties". Journal of Medicinal Chemistry. 46 (11): 2110–6. doi:10.1021/jm020329q. PMID 12747783.
- ↑ Huffman, J. W.; Padgett, L. W. (2005). "Recent Developments in the Medicinal Chemistry of Cannabimimetic Indoles, Pyrroles and Indenes". Current Medicinal Chemistry. 12 (12): 1395–1411. doi:10.2174/0929867054020864. PMID 15974991.
- ↑ Manera, C.; Tuccinardi, T.; Martinelli, A. (2008). "Indoles and Related Compounds as Cannabinoid Ligands". Mini Reviews in Medicinal Chemistry. 8 (4): 370–387. doi:10.2174/138955708783955935. PMID 18473928.
- ↑ Adam, J. M.; et al. (2010). "Design, synthesis, and structure–activity relationships of indole-3-carboxamides as novel water soluble cannabinoid CB1 receptor agonists". MedChemComm. Royal Society of Chemistry. 1: 54. doi:10.1039/c0md00022a.
- ↑ Kiyoi T, et al. (August 2010). "Design, synthesis, and structure-activity relationship study of conformationally constrained analogs of indole-3-carboxamides as novel CB1 cannabinoid receptor agonists". Bioorganic & Medicinal Chemistry Letters. 20 (16): 4918–21. doi:10.1016/j.bmcl.2010.06.067. PMID 20634067.
- ↑ Moir EM, et al. (December 2010). "Design, synthesis, and structure-activity relationship study of bicyclic piperazine analogs of indole-3-carboxamides as novel cannabinoid CB1 receptor agonists". Bioorganic & Medicinal Chemistry Letters. 20 (24): 7327–30. doi:10.1016/j.bmcl.2010.10.061. PMID 21074434.
- ↑ Blaazer, A. R.; et al. (2011). "Novel indole and azaindole (pyrrolopyridine) cannabinoid (CB) receptor agonists: Design, synthesis, structure–activity relationships, physicochemical properties and biological activity". European Journal of Medicinal Chemistry. 46 (10): 5086–5098. doi:10.1016/j.ejmech.2011.08.021. PMID 21885167.
Further reading
- John Hynes., et al. C3 AMIDO-INDOLE CANNABINOID RECEPTOR MODULATORS. Bioorganic and Medical Chemistry Letters. Volume 12 issue 17, 2 September 2002 pages 2399-2402
- Frost, J. M., et al. (2010). "Indol -3-ylcycloalkyl Ketones: Effects of N1 Substituted Indole Side Chain Variations on CB2 Cannabinoid Receptor Activity". Journal of Medicinal Chemistry 53 (1): 295. doi :10.1021/ jm901214q. PMID 19921781
- Chin CL, et al. (January 2008). "Differential effects of cannabinoid receptor agonists on regional brain activity
using pharmacological MRI". British Journal of Pharmacology 153 (2): 367–79. doi :10.1038/ sj.bjp .0707506. PMC 2219521. PMID 17965748