Nitrosonium
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Nitrosonium | |
| Systematic IUPAC name
Iminooxidanium | |
| Identifiers | |
| 14452-93-8 | |
| 3D model (Jmol) | Interactive image |
| Abbreviations | NO(+) |
| ChEBI | CHEBI:29120 |
| ChemSpider | 76569 |
| 456 | |
| PubChem | 84878 |
| |
| |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
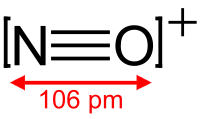
The nitrosonium ion is NO+, in which the nitrogen atom is bonded to an oxygen atom with a bond order of 3, and the overall diatomic species bears a positive charge. This ion is usually obtained as the following salts: NOClO4, NOSO4H (nitrosylsulfuric acid, more descriptively written ONSO3OH), and NOBF4. The ClO−
4 and BF−
4 salts are slightly soluble in CH3CN. NOBF4 can be purified by sublimation at 200–250 °C and 0.01 mmHg (1.3 Pa).
NO+ is isoelectronic with CO, CN− and N2. It arises via protonation of nitrous acid:
- HONO + H+ ⇌ NO+ + H2O
Chemical Properties
Hydrolysis
NO+ reacts readily with water to form nitrous acid:
- NOBF4 + H2O → HONO + HBF4
For this reason, NOBF4 must be protected from water or even moist air. With base, the reaction generates nitrite:
- NOBF4 + 2 NaOH → NaNO2 + NaBF4 + H2O
As a diazotizing agent
NO+ reacts with aryl amines, ArNH2, to give diazonium salts, ArN+
2. The resulting diazonium group is easily displaced (unlike the amino group) by a variety of nucleophiles.
As an oxidizing agent
NO+, e.g. as NOBF4, is a strong oxidizing agent:[1]
- vs. ferrocene/ferrocenium, [NO]+ in CH2Cl2 solution has a redox potential of 1.00 V (or 1.46–1.48 V vs SCE)
- vs. ferrocene/ferrocenium, [NO]+ in CH3CN solution has a redox potential of 0.87 V vs. (or 1.27–1.25 V vs SCE)
NOBF4 is a convenient oxidant because the byproduct NO is a gas, which can be swept from the reaction using a stream of N2. Upon contact with air, NO forms NO2, which can cause secondary reactions if it is not removed. NO2 is readily detectable by its characteristic orange color.
Nitrosylation of arenes
Electron-rich arenes are nitrosylated using NOBF4.[2] One example involves anisole:
- CH3OC6H5 + NOBF4 → CH3OC6H4-4-NO + HBF4
Nitrosonium, NO+, is sometimes confused with nitronium, NO+
2, the active agent in nitrations. These species are quite different, however. Nitronium is a more potent electrophile than is nitrosonium, as anticipated by the fact that the former is derived from a strong acid (nitric acid) and the latter from a weak acid (nitrous acid).
As a source of nitrosyl complexes
NOBF4 reacts with some metal carbonyl complexes to yield related metal nitrosyl complexes.[3] One must be careful that [NO]+ is transferred vs. electron transfer (see above).
- (C6Et6)Cr(CO)3 + NOBF4 → [(C6Et6)Cr(CO)2(NO)]BF4 + CO
See also
References
- ↑ N. G. Connelly, W. E. Geiger (1996). "Chemical Redox Agents for Organometallic Chemistry". Chem. Rev. 96 (2): 877–910. doi:10.1021/cr940053x. PMID 11848774.
- ↑ E. Bosch and J. K. Kochi, "Direct Nitrosation of Aromatic Hydrocarbons and Ethers with the Electrophilic Nitrosonium Cation" Journal of Organic Chemistry, 1994, volume 59, pp. 5573–5586.
- ↑ T. W. Hayton, P. Legzdins, W. B. Sharp "Coordination and Organometallic Chemistry of Metal-NO Complexes" Chemical Reviews 2002, volume 102, pp. 935–991
