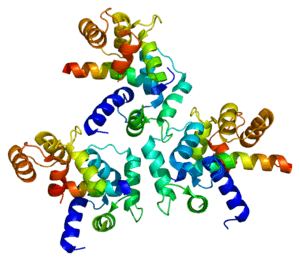
L-type calcium channel
| Calcium channel, voltage-dependent | |
|---|---|
|
Crystallographic structure | |
| Identifiers | |
| Symbol | Calcium channel, voltage-dependent |
The L-type calcium channel (also known as the dihydropyridine channel, or DHP channel) is part of the high-voltage activated family of voltage-dependent calcium channel.[2] "L" stands for long-lasting referring to the length of activation. This channel has four subunits (Cav1.1, Cav1.2, Cav1.3, Cav1.4).
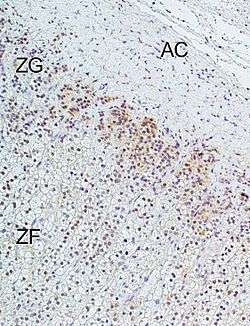
L-type calcium channels are responsible for the excitation-contraction coupling of skeletal, smooth, cardiac muscle, and for aldosterone secretion in endocrine cells of the adrenal cortex.[1]
In cardiac myocytes, the L-type calcium channel passes inward Ca2+ current and triggers calcium release from the sarcoplasmic reticulum by activating ryanodine receptor 2 (RyR2) (calcium-induced-calcium-release).[3] Phosphorylation of these channels increases their permeability to calcium and increases the contractility of their respective cardiac myocytes.
L-type calcium channel blocker drugs are used as cardiac antiarrhythmics or antihypertensives, depending on whether the drugs have higher affinity for the heart (the phenylalkylamines, like verapamil), or for the vessels (the dihydropyridines, like nifedipine).
In skeletal muscle, there is a very high concentration of L-type calcium channels, situated in the T-tubules. Muscle depolarization results in large gating currents, but anomalously low calcium flux, which is now explained by the very slow activation of the ionic currents. For this reason, little or no Ca2+ passes across the T-tubule membrane during a single action potential.
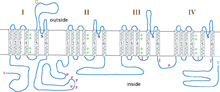
Structure
Like most voltage-gated ion channels, the α-subunit is composed of 4 subunits. Each subunit is formed by 6 alpha-helical, transmembrane domains that cross the membrane (numbered S1-S6). The S1-S4 subunits make up the voltage sensor, while S5-S6 subunits make up the selectivity filter.[4]
Genes
See also
References
- 1 2 Felizola SJ, Maekawa T, Nakamura Y, Satoh F, Ono Y, Kikuchi K, Aritomi S, Ikeda K, Yoshimura M, Tojo K, Sasano H (2014). "Voltage-gated calcium channels in the human adrenal and primary aldosteronism.". J Steroid Biochem Mol Biol. 144 (part B): 410–416. doi:10.1016/j.jsbmb.2014.08.012. PMID 25151951.
- ↑ Rossier, Michel F. (2016). "T-Type Calcium Channel: A Privileged Gate for Calcium Entry and Control of Adrenal Steroidogenesis". Frontiers in Endocrinology. 7. doi:10.3389/fendo.2016.00043. ISSN 1664-2392.
- ↑ Yamakage M, Namiki A (2002). "Calcium channels — basic aspects of their structure, function and gene encoding; anesthetic action on the channels — a review". Can J Anaesth 49 (2): 151–64. doi:10.1007/BF03020488. PMID 11823393.
- ↑ Catterall, William A.; Perez-Reyes, Edward; Snutch, Terrance P.; Striessnig, Joerg (December 2005). "International Union of Pharmacology. XLVIII. Nomenclature and Structure-Function Relationships of Voltage-Gated Calcium Channels". Pharmacol Rev. 57 (4): 411–425. doi:10.1124/pr.57.4.5. PMID 16382099. Retrieved 30 November 2014.
External links
- "Voltage-Gated Calcium Channels". IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology.
- L-Type Calcium Channel at the US National Library of Medicine Medical Subject Headings (MeSH)