Melanotan II
 | |
| Names | |
|---|---|
| Other names
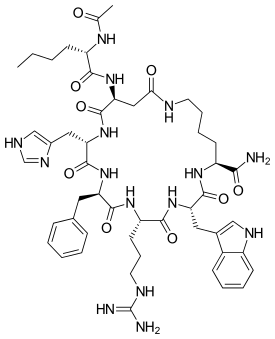
Ac-Nle-cyclo[Asp-His-D-Phe-Arg-Trp-Lys]-NH2
PT-14 | |
| Identifiers | |
| 121062-08-6 | |
| ChEMBL | ChEMBL430239 |
| ChemSpider | 83450 |
| 1323 | |
| MeSH | melanotan-II |
| PubChem | 92432 |
| |
| Properties | |
| C50H69N15O9 | |
| Molar mass | 1024.180 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
- Note: this article describes melanotan II which should not be confused with melanotan I which is also known by the standardized name afamelanotide.
Melanotan II (![]() i/mɛˈlænoʊtæn/) is a synthetic analogue of the naturally-occurring melanocortin peptide hormone α-melanocyte-stimulating hormone (α-MSH) that in usage has been shown to produce melanogenesis (part of the tanning process that produces darkening pigmentation in the skin) and have aphrodisiac effects in preliminary studies and clinical trials.[1][2][3] Developed at the University of Arizona, it is a cyclic lactam analogue of α-MSH with the amino acid sequence Ac-Nle-cyclo[Asp-His-D-Phe-Arg-Trp-Lys]-NH2.
i/mɛˈlænoʊtæn/) is a synthetic analogue of the naturally-occurring melanocortin peptide hormone α-melanocyte-stimulating hormone (α-MSH) that in usage has been shown to produce melanogenesis (part of the tanning process that produces darkening pigmentation in the skin) and have aphrodisiac effects in preliminary studies and clinical trials.[1][2][3] Developed at the University of Arizona, it is a cyclic lactam analogue of α-MSH with the amino acid sequence Ac-Nle-cyclo[Asp-His-D-Phe-Arg-Trp-Lys]-NH2.
As of 2016, no product containing melanotan II has ever received approval for use from any governmental drug regulatory bodies outside of sanctioned animal and human clinical trials. Unlicensed and untested powders sold as "melanotan II" are found on the Internet[4] and are reported to be used by thousands of members of the general public for sunless tanning. Multiple regulatory bodies have warned consumers the peptides may be unsafe and ineffective in usage with one regulatory agency warning that consumers who purchase any product labeled "melanotan" risk buying a counterfeit drug.[5] Medical researchers and Clinuvel Pharmaceuticals, the company developing the related peptide afamelanotide (also known as melanotan I), have warned consumers that counterfeit products sold using the names "melanotan I and II", "pose a hazard to public health".[6]
Mechanism of action
Melanotan II acts as a non-selective agonist of the melanocortin receptors, MC1, MC3, MC4, MC5.[7][8] Like other melanocortinergic peptides, such as the melanocyte-stimulating hormones (MSHs) and afamelanotide, but unlike adrenocorticotropic hormone (ACTH), melanotan II lacks affinity for the MC2 receptor.[7][8] Reported activity of the drug is as follows:[9]
The melanogenesis produced by melanotan II is caused by activation of the MC1 receptor, whereas its sexual effects are thought to be related to its ability to activate the MC4 receptor (though the MC3 is thought to possibly also be involved).[7][8][10][11] Other effects of melanotan II, mostly regarded as adverse effects, include flushing, nausea, vomiting, stretching, yawning, and loss of appetite (the last via activation of MC4).[12][13]
Melanotan II produces bremelanotide, which, comparatively, is reported to be approximately 50-fold more potent as an inducer of sexual arousal in rats, as an active metabolite.[14]
Historical development
Melanotan II was first synthesized at the University of Arizona. Researchers there knew that one of the best defenses against skin cancer was melanin activated in the skin, a tan. They hypothesized that an effective way to reduce skin cancer rates in people would be to induce the body's natural pigmentary system to produce a protective tan prior to UV exposure. The body's naturally-occurring hormone α-MSH causes melanogenesis, a process by which the skin's pigment cells (melanocytes) produce the skin's pigment (melanin).[15] They tested to see if administering this endogenous hormone to the body directly could be an effective method to cause sunless tanning. What they found was that while it appeared to work, natural α-MSH had too short a half-life in the body to be practical as a therapeutic drug. So they decided to find a more potent and stable alternative, one that would be more practical.
After synthesizing and screening hundreds of molecules, the researchers headed by Victor J. Hruby and Mac E. Hadley,[16] found a peptide, [Nle4,D-Phe7]-α-MSH, that was approximately 1,000 times more potent than natural α-MSH.[17] They dubbed this new peptide molecule "melanotan" (later melanotan-1, now known as afamelanotide). They subsequently developed another analogue, Ac-Nle-cyclo[Asp-His-D-Phe-Arg-Trp-Lys]-NH2), which they called "melanotan II". The scientists hoped to use these peptides to prevent melanoma by stimulating the body's natural pigmentary mechanism to create a tan without first needing exposure to harmful levels of UV radiation.[18] This in turn, they hypothesized, could reduce the potential for skin damage[19] that can possibly lead to skin cancer.
The scientists licensed their patented peptides, via a technology transfer company, to a number of biotechnology companies who intend to develop them into drugs.[20]
Human clinical trials
A pilot Phase I clinical trial conducted on three males by the College of Medicine, Pharmacology Department, University of Arizona in Tucson, Arizona published in 1996 reported that, "Melanotan II has tanning activity in humans given only 5 low doses every other day by subcutaneous injection." The side effects reported were mild nausea and a "stretching and yawning complex" that correlated with spontaneous penile erections.[21]
The Department of Pharmacology, University of Arizona College of Medicine published a study in 1998 that involved ten men who suffered from psychogenic erectile dysfunction. Their trial concluded that, "Melanotan-II is a potent initiator of erections in men with psychogenic erectile dysfunction and has manageable side effects at a dose of 0.025 mg./kg."[22]
A clinical study published in 2000 of 20 men with psychogenic and organic erectile dysfunction conducted at the Section of Urology of The University of Arizona College of Medicine concluded, "that Melanotan II is a potent initiator of penile erection in men with erectile dysfunction."[23]
General population usage of melanotan peptides
General public users of the melanotan peptides have been reported to number into the thousands with one BBC report explaining that a January 2009 straw poll by the reporting journalist of just 6 UK needle exchanges revealed, "nearly 500 people wanting the syringes [for peptide usage] or information about melanotan".[24][25][26] Academic researchers reported on a then "thriving" internet community of users of the peptides at the site Melanotan.org where members discussed their experiences using the unlicensed and unregulated drugs.[27] The site was reported to number more than 5,000 members as of February 2009.[28] A January 2009 report in Wired Science described the site's forum as having more than 50,000 posts primarily covering "Usage and Experimentation" by members with many covering detailed regimens on how to attain skin darkening and/or sexual function [with Melanotan II] improvements.[29]
In May 2010 the Norwegian tabloid daily Verdens Gang published a story based upon a report by the Norwegian Pharmacy Association stating that 10,000 syringes are sold annually to Norwegian users of melanotan-1 and melanotan II.[30]
"Melanotan" products sold for human use
A number of products are sold online and in gyms and beauty salons as "melanotan" or "melanotan-1" which purport to have the same chemical make up as afamelanotide, of which the visual effect resulting from usage has been noted in an article by Wired.com as being "eerily similar to results obtained in trials at the University of Arizona or by Clinuvel [An Australian company trialing the related afamelanotide peptide]". The Wired.com article explained that these products were Melanotan II, "a similar (but not identical) compound"[29] and Clinuvel has stated that products sold as "melanotan" are "illegal" and "wholly unrelated to Clinuvel's proprietary afamelanotide".[6] Chemicals sold as "melanotan" are not illegal to import, use or own, however their domestic sales (non-export) and supply for human use outside of government sanctioned clinical trials is illegal within the boundaries of most jurisdictions, including the UK,[24] USA, Europe and Australia.[31]
A 2009 paper on unlicensed "melanotan" products, sold on the internet, has reported that the products caused moles to darken and increase in size over a short period, "an early warning sign of skin cancer".[32][33][34][35] Academic researchers at Liverpool John Moores University specialized in performance-enhancing drugs published an editorial in the British Medical Journal suggesting that use of 'melanotan I and II', "could damage the immune and cardiovascular systems as well as triggering other problems".[36]
Health warnings on "melanotan" products sold for human use
In 2007, the FDA issued a Warning Letter to an American online vendor illegally marketing melanotan II on the internet as a drug that prevents skin cancer and assists tanning. The FDA has not licensed melanotan II, and explained: "There is no evidence that the product is generally recognized as safe and effective [GRAS/E] for its labeled uses." The FDA concurrently gave a blanket warning advising consumers to, "stop using Melanotan II, an unapproved product".[37]
On August 8, 2008 the Danish Medicines Agency (DMA) issued a warning against the usage of any product called "Melanotan" purchased on the internet, noting that claims that imply that it has an, "effect" for protection against skin cancer, "has not been documented". The DMA further warned that Melanotan has not undergone tests for its effect and possible side effects, and is an "illegal medicinal product" that it is not licensed for usage in the EU or the USA.[38]
The UK Medicines and Healthcare products Regulatory Agency issued a similar warning on November 17, 2008 stating that "We are warning people not to use this product. Don’t be fooled into thinking that Melanotan offers a shortcut to a safer and more even tan. The safety of these products is unknown and they are unlicensed in the UK. The side effects could be extremely serious. If you have used either of these products do not use them again and if you have any concerns you should seek advice from your doctor."[39]
The FDA issued a warning letter to another online vendor in January 2009 selling "10 MG MT 2 [Melanotan II] -American Lab" as advertisements of the products on their website were in breach of the Federal Food, Drug, and Cosmetic Act as the site was selling "new drug" products "intended for human use".[40]
The Irish Medicines Board (IMB) issued a precautionary safety alert on February 27, 2009 regarding the use of "the unauthorised medicine Melanotan (I and II)", sold as a powder for injection, stating that "Melanotan is not authorised in Ireland and therefore the IMB cannot guarantee the efficacy, safety or quality of this product."[4] In its release, the IMB announced that its tests had found the presence of microbial contamination in a vial of water sold together with melanotan powder which "would expose recipients to a risk of serious infection".
The Norwegian Medicines Agency has issued warnings in 2007[41] and 2009[5] about the use of "Melanotan" sold online, while a spokesman for the Australian Therapeutic Goods Administration warned consumers to be "very wary" of using it.[31]
In July 2010, the Swedish Medical Products Agency issued a warning against the usage of melanotan II.[42]
Bremelanotide
Palatin Technologies developed another hormone targeted towards sexual dysfunction therapy based upon melanotan II called bremelanotide (formerly PT-141). Bremelanotide is a deaminated active metabolite of melanotan II that lacks the C-terminal amide function. The drug has been undergoing clinical trials for the treatment of hypoactive sexual desire disorder and erectile dysfunction. It is intended for both men and women. Preliminary results have proven the efficacy of this drug,[43] however development was briefly suspended[44] due to a side effect of increased blood pressure observed in a small number of trial subjects administered the drug intranasally. On August 12, 2009, Palatin announced positive results (none of the previous heightened blood pressure effects were observed) of a phase I clinical study where trial subjects were instead administered the drug subcutaneously.[45] As of December 2014, the subcutaneous formulation of bremelanotide is in phase III clinical trials for sexual dysfunction. In addition, Palatin is concurrently developing PL-6983 for sexual dysfunction, a related, more selective drug that has been shown to produce lower increases in blood pressure in animal models relative to bremelanotide.[46]
References
- ↑ Hadley ME (Oct 2005). "Discovery that a melanocortin regulates sexual functions in male and female humans". Peptides. 26 (10): 1687–9. doi:10.1016/j.peptides.2005.01.023. PMID 15996790.
- ↑ "Tanning drug may find new life as Viagra alternative". CNN. 1999. Retrieved 2008-06-12.
- ↑ Jaroff, Leon (1999-06-20). "Tanning Bonus". Time. Retrieved 2008-09-17.
- 1 2 "Melanotan Powder for Injection". Notice Information: - Warning - 27/02/2009. Irish Medicines Board. 2009. Retrieved 2009-02-02.
- 1 2 "Melanotan – farlig og ulovlig brunfarge". Norwegian Medicines Agency. 2009-01-23. Retrieved 2009-03-11.
- 1 2 Clinuvel Position Statement on counterfeit products. February 10, 2009.
- 1 2 3 Yang Y, Chen M, Lai Y, Gantz I, Georgeson KE, Harmon CM (2002). "Molecular determinants of human melanocortin-4 receptor responsible for antagonist SHU9119 selective activity". J. Biol. Chem. 277 (23): 20328–35. doi:10.1074/jbc.M201343200. PMID 11912210.
- 1 2 3 Wikberg, Jarl ES (2001). "Melanocortin receptors: new opportunities in drug discovery". Expert Opinion on Therapeutic Patents. 11 (1): 61–76. doi:10.1517/13543776.11.1.61. ISSN 1354-3776.
- ↑ Schiöth, Helgi B; Mutulis, Felikss; Muceniece, Ruta; Prusis, Peteris; Wikberg, Jarl E S (1998). "Discovery of novel melanocortin4receptor selective MSH analogues". British Journal of Pharmacology. 124 (1): 75–82. doi:10.1038/sj.bjp.0701804. ISSN 0007-1188.
- ↑ David O. Norris; Kristin H. Lopez (25 November 2010). Hormones and Reproduction of Vertebrates. Academic Press. pp. 4–. ISBN 978-0-08-095809-5.
- ↑ King SH, Mayorov AV, Balse-Srinivasan P, Hruby VJ, Vanderah TW, Wessells H (2007). "Melanocortin receptors, melanotropic peptides and penile erection". Curr Top Med Chem. 7 (11): 1098–1106. doi:10.2174/1568026610707011111. PMC 2694735
 . PMID 17584130.
. PMID 17584130. - ↑ Tony M. Plant; Anthony J. Zeleznik (15 November 2014). Knobil and Neill's Physiology of Reproduction: Two-Volume Set. Academic Press. pp. 2230–. ISBN 978-0-12-397769-4.
- ↑ Alan J. Wein; Louis R. Kavoussi; Andrew C. Novick; Alan W. Partin, Craig A. Peters (28 September 2011). Campbell-Walsh Urology. Elsevier Health Sciences. pp. 743–. ISBN 1-4557-2298-7. Cite uses deprecated parameter
|coauthors=(help) - ↑ US patent 6579968, Christine H. Blood; Annette M. Shadiack & Joanna K. Bernstein et al., "Compositions and methods for treatment of sexual dysfunction", published 17 June 2003, assigned to Palatin Technologies, Inc.
- ↑ Parrish JA, Jaenicke KF, Anderson RR (1982). "Erythema And Melanogenesis Action Spectra Of Normal Human Skin". Photochemistry and Photobiology. 36 (2): 187–191. doi:10.1111/j.1751-1097.1982.tb04362.x. PMID 7122713.
- ↑ Kolata, Gina (1987-11-24). "Futuristic Treatments For the Hair and Skin". The New York Times. Retrieved 2010-04-20.
- ↑ "UA-developed synthetic hormones speed a tan". Arizona Daily Star. 2006. Retrieved 2009-07-26.
- ↑ "The Barbie Drug". Campbell Live, TV3 (New Zealand)V3. 2007. Archived from the original (FLV) on 4 October 2011. Retrieved 2007-09-16.
- ↑ Christian Mahne (2002-09-04). "Implant offers tanning revolution". BBC. Archived from the original (RealVideo) on 8 September 2014. Retrieved 2009-07-26.
- ↑ Johnson, Johnnie D (26 July 2005). "Competitive Technologies Licenses Additional Patent to Melanotan Corporation, Receives Additional Shares of Epitan". Bloomberg Business. Retrieved 1 July 2015.
- ↑ Dorr RT, Lines R, Levine N, et al. (1996). "Evaluation of melanotan-II, a superpotent cyclic melanotropic peptide in a pilot phase-I clinical study". Life Sci. 58 (20): 1777–84. doi:10.1016/0024-3205(96)00160-9. PMID 8637402.
- ↑ Wessells H, Fuciarelli K, Hansen J, et al. (Aug 1998). "Synthetic melanotropic peptide initiates erections in men with psychogenic erectile dysfunction: double-blind, placebo controlled crossover study". J Urol. 160 (2): 389–93. doi:10.1016/S0022-5347(01)62903-3. PMID 9679884.
- ↑ Wessells H, Levine N, Hadley ME, Dorr R, Hruby V (Oct 2000). "Melanocortin receptor agonists, penile erection, and sexual motivation: human studies with Melanotan II". Int J Impot Res. 12 (Suppl 4): S74–9. doi:10.1038/sj.ijir.3900582. PMID 11035391.
- 1 2 "Illegal Tanning drugs sold" (Windows Media Video). Inside Out, BBC. 2009-01-14. Retrieved 2009-07-23.
- ↑ "Believe It Or Not 'Tanorexia' A Very Real Problem". WCBS-TV, CBS. 2009-05-20. Archived from the original (Flash Video) on 21 May 2009. Retrieved 2009-07-23.
- ↑ "Fools Gold". Cosmopolitan (Australia). 2009-06-14. Retrieved 2009-07-25.
- ↑ Kim Mulvihill (2009-03-05). "'Barbie drug' promises instant tan without sun damage". KSL-TV, NBC. Archived from the original (Flash Video) on 4 June 2011. Retrieved 2015-10-20.
- ↑ Devlin, Kate (2009-02-17). "People risking health with internet 'tan jabs', warns expert". London: The Daily Telegraph. Retrieved 2009-07-23.
- 1 2 Madrigal, Alexis (2009-01-29). "Suntan Drug Greenlighted for Trials". Wired. Retrieved 2009-04-11.
- ↑ Flåm, Karoline (2010-05-29). "Apoteket selger årlig 10.000 sprøyter til barbie-dopet". Norway: Verdens Gang. Retrieved 2010-07-08. (Norwegian)
- 1 2 "Tanning drug a health risk". Herald Sun. 2009-10-31. Retrieved 2009-10-31.
- ↑ Ewan A Langan; Z. Nie; Lesley E Rhodes (June 2010). "Melanotropic peptides: More than just "Barbie drugs" and "sun tan jabs?"". British Journal of Dermatology. 163 (3): 451–5. doi:10.1111/j.1365-2133.2010.09891.x. PMID 20545686.
- ↑ Ewan A Langan; Denise Ramlogan; Lynne A Jamieson; Lesley E Rhodes (January 2009). "Change in moles linked to use of unlicensed "sun tan jab"". BMJ. 388: b277. doi:10.1136/bmj.b277. PMID 19174439.
- ↑ "Worry over tan jab mole changes". BBC. 2009-01-28. Retrieved 2009-03-04.
- ↑ Devlin, Kate (2009-01-27). "Illegal tanning drug can change appearance of moles, scientists warn". London: The Daily Telegraph. Retrieved 2009-03-04.
- ↑ "Risky tan jab warnings 'ignored'". BBC. 2009-02-18. Retrieved 2009-03-04.
- ↑ "FDA Warns About Unapproved Product, Melanotan II". U.S. Food and Drug Administration. 2007. Retrieved 2009-07-31.
- ↑ "Warning against the product Melanotan". Danish Medicines Agency. 2008. Retrieved 2008-08-11.
- ↑ ""Tan jab" is an unlicensed medicine and may not be safe". MHRA. 2008. Retrieved 2008-11-17.
- ↑ "US Lab Research Inc Warning letter". FDA. 2009-01-29. Retrieved 2009-06-23.
- ↑ "Legemiddelverket advarer mot bruk av Melanotan". Norwegian Medicines Agency. 2007-12-13. Retrieved 2009-03-11.
- ↑ "Läkemedelsverket varnar för användning av Melanotan II". Medical Products Agency. 2010-07-26. Retrieved 2010-07-26. (Swedish)
- ↑ King, S.H.; Mayorov AV; Balse-Srinivasan P; Hruby VJ; Vanderah TW; Wessells H. (2007). "Melanocortin Receptors, Melanotropic Peptides and Penile Erection". Curr Top Med Chem. 7 (11): 1098–1106. doi:10.2174/1568026610707011111. PMC 2694735
 . PMID 17584130.
. PMID 17584130. - ↑ "Palatin Technologies Announces New Strategic Objectives". Retrieved 2008-05-13.
- ↑ "PALATIN TECHNOLOGIES, INC. REPORTS POSITIVE BREMELANOTIDE STUDY; IMPROVED SAFETY PROFILE WITH SUBCUTANEOUS ADMINISTRATION". Retrieved 2009-08-12.
- ↑ Natasha Mitchner (2009). Hypoactive Sexual Desire Disorder in Women: Implications for Family Practice (PDF). Dowden Health Media.