Lead(II) fluoride
| | |
 | |
| Names | |
|---|---|
| Other names
Lead difluoride plumbous fluoride | |
| Identifiers | |
| 7783-46-2 | |
| ECHA InfoCard | 100.029.089 |
| PubChem | 24549 |
| Properties | |
| PbF2 | |
| Molar mass | 245.20 g/mol |
| Appearance | white powder |
| Odor | odorless |
| Density | 8.445 g/cm3 (orthorhombic) 7.750 g/cm3 (cubic) |
| Melting point | 824 °C (1,515 °F; 1,097 K) |
| Boiling point | 1,293 °C (2,359 °F; 1,566 K) |
| 0.057 g/100 mL (0 °C) 0.0671 g/100 mL (20 °C)[1] | |
| Solubility product (Ksp) |
2.05 x 10−8 (20 °C) |
| Solubility | soluble in nitric acid; insoluble in acetone and ammonia |
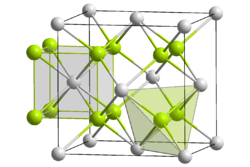
| Structure | |
| Fluorite (cubic), cF12 | |
| Fm3m, No. 225 | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
3031 mg/kg (oral, rat) |
| Related compounds | |
| Other anions |
Lead(II) chloride Lead(II) bromide Lead(II) iodide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Lead(II) fluoride (PbF2) is a chemical compound that is an odorless white solid.
Conditions/substances to avoid are: strong oxidizers.
Uses
Lead(II) fluoride is used:
- in low melting glasses
- in glass coatings to reflect infrared rays
- in phosphors for television-tube screens
- as a catalyst for the manufacture of picoline
Preparation
Lead(II) fluoride can be prepared by several methods. It is obtained by treating lead(II) hydroxide or lead(II) carbonate with hydrofluoric acid, followed by evaporation of the solution:
- Pb(OH)2 + 2 HF → PbF2 + 2 H2O
Alternatively, it is precipitated by adding hydrofluoric acid to a lead(II) salt solution, or by adding potassium fluoride to a lead(II) nitrate solution.[2]
- 2 KF + Pb(NO3)2 → PbF2 + 2 KNO3
References
- ↑ NIST-data review 1980
- ↑ Arnold Hollemann, Egon Wiberg, 101st ed., de Gruyter 1995 Berlin; ISBN 3-11-012641-9
This article is issued from Wikipedia - version of the 6/25/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.