Platinum hexafluoride
 | |
| Names | |
|---|---|
| IUPAC name
Platinum hexafluoride | |
| Other names
Platinum(VI) fluoride | |
| Identifiers | |
| 13693-05-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 21106464 |
| ECHA InfoCard | 100.033.816 |
| |
| |
| Properties | |
| PtF6 | |
| Molar mass | 309.07 g/mol |
| Appearance | dark-red crystals |
| Density | 3.83 g/cm3 |
| Melting point | 61.3 °C (142.3 °F; 334.4 K) |
| Boiling point | 69.14 °C (156.45 °F; 342.29 K) |
| reacts violently | |
| Structure | |
| Orthorhombic, oP28 | |
| Pnma, No. 62 | |
| octahedral (Oh) | |
| 0 | |
| Hazards | |
| Main hazards | oxidizer |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
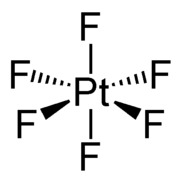
Platinum hexafluoride is the chemical compound with the formula PtF6. It is a dark-red volatile solid that forms a red gas. The compound is a unique example of platinum in the +6 oxidation state. With only four d-electrons, it is paramagnetic with a triplet ground state. PtF6 is a strong oxidant and a strong fluorinating agent. PtF6 is octahedral in both the solid state and in the gaseous state. The Pt-F bond lengths are 185 picometers.[1]
Synthesis
PtF6 was first prepared by reaction of fluorine with platinum metal.[2] This route remains the method of choice.[1]
- Pt + 3 F2 → PtF6
PtF6 can also be prepared by disproportionation of the pentafluoride (PtF5), with the tetrafluoride (PtF4) as a byproduct. The required PtF5 can be obtained by fluorinating PtCl2:
- 2 PtCl2 + 5 F2 → 2 PtF5 + 2 Cl2
- 2 PtF5 → PtF6 + PtF4
Hexafluoroplatinates
Platinum hexafluoride can gain an electron to form the hexafluoroplatinate anion, PtF−
6. It is formed by reacting platinum hexafluoride with relatively uncationisable elements and compounds, for example with xenon to form "XePtF
6" (actually a mixture of XeFPtF
5, XeFPt
2F
11, and Xe
2F
3PtF
6), known as xenon hexafluoroplatinate. The discovery of this reaction in 1962 proved that noble gases form chemical compounds. Previous to the experiment with xenon, PtF
6 had been shown to react with oxygen to form [O2]+[PtF6]−, dioxygenyl hexafluoroplatinate.
See also
References
- 1 2 Drews, T.; Supel, J.; Hagenbach, A.; Seppelt, K. "Solid State Molecular Structures of Transition Metal Hexafluorides" Inorganic Chemistry 2006, volume 45, pp 3782-3788.doi:10.1021/ic052029f
- ↑ Weinstock, B.; Claassen, H. H.; Malm, J. G. (1957). "Platinum Hexafluoride". Journal of the American Chemical Society. 79: 5832–5832. doi:10.1021/ja01578a073.
General reading
- Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.