Plumbane
 | |
| Names | |
|---|---|
| IUPAC name
Plumbane | |
| Other names
Plumbane, lead tetrahydride, tetrahydridolead, lead(IV) hydride | |
| Identifiers | |
| 15875-18-0 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:30181 |
| ChemSpider | 109888 |
| PubChem | 123278 |
| |
| |
| Properties | |
| PbH4 | |
| Molar mass | 211.23 g/mol |
| Boiling point | −13 °C (9 °F; 260 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
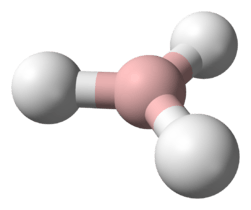
Plumbane, PbH4, is a metal hydride and group 14 hydride composed of lead and hydrogen.[1] Plumbane is not well-characterized or well-known, and it is thermodynamically unstable with respect to the loss of a hydrogen atom.[2] Derivatives of plumbane include lead tetrafluoride, (PbF4), and tetraethyllead, ((CH3CH2)4Pb).
History
Until recently, it was uncertain whether plumbane had ever actually been synthesized;[3] although, the first reports date back to the 1920s[4] and in 1963, Saalfeld and Svec reported the observation of PbH+
4 by mass spectrometry.[5] Plumbane has repeatedly been the subject of Dirac–Hartree–Fock relativistic calculation studies, which investigate the stabilities, geometries, and relative energies of hydrides of the formula MH4 or MH2.[2][6][7]
Properties
Plumbane is an unstable colorless gas and is the heaviest group IV hydride.[8] Furthermore, plumbane has a tetrahedral (Td) structure with an equilibrium distance between lead and hydrogen of 1.73 Å.[9] By weight percent, the composition of plumbane is 1.91% hydrogen and 98.09% lead. In plumbane, the formal oxidation states of hydrogen and lead are -1 and +4, respectively, because the electronegativity of hydrogen is higher than that of lead. The stability of metal hydrides with the formula MH4 (M = C–Pb) decreases as the atomic number of M increases.
Preparation
In general, metal hydrides are formed when hydrogen gas is allowed to react with metals. They can serve to store hydrogen gas, which is highly flammable.
Early studies of PbH4 revealed that the molecule is unstable as compared to its lighter congeners (silane, germane, and stannane).[10] It cannot be made by methods used to synthesize GeH4 or SnH4.
Recently, plumbane has been synthesized from lead(II) nitrate, Pb(NO3)2, and sodium borohydride, NaBH4.[11] Additionally, a non-nascent mechanism for plumbane synthesis has been developed.[12]
In 2003, Wang and Andrews carefully studied the preparation of PbH4 by laser ablation and additionally identified the infrared (IR) bands.[13]
Congeners
Congeners of plumbane include:
References
- ↑ Porritt, C. J. (1975). Chem. Ind-London. 9: 398. Missing or empty
|title=(help) - 1 2 Hein, Thomas A.; Thiel, Walter; Lee, Timothy J. (1993). "Ab initio study of the stability and vibrational spectra of plumbane, methylplumbane, and homologous compounds". The Journal of Physical Chemistry. 97 (17): 4381–4385. doi:10.1021/j100119a021.
- ↑ Cotton, F. A.; Wilkinson, G.; Murillo, C. A.; Bochman, M. Advanced Inorganic Chemistry. Wiley: New York, 1999
- ↑ Paneth, Fritz; Nörring, Otto (1920). "Über Bleiwasserstoff". Berichte der deutschen chemischen Gesellschaft (A and B Series). 53 (9): 1693–1710. doi:10.1002/cber.19200530915.
- ↑ Saalfeld, Fred E.; Svec, Harry J. (1963). "The Mass Spectra of Volatile Hydrides. I. The Monoelemental Hydrides of the Group IVB and VB Elements". Inorganic Chemistry. 2: 46–50. doi:10.1021/ic50005a014.
- ↑ Desclaux, J. P.; Pyykko, P. (1974). "Relativistic and non-relativistic Hartree-Fock one-centre expansion calculations for the series CH4 to PbH4 within the spherical approximation". Chemical Physics Letters. 29 (4): 534–539. doi:10.1016/0009-2614(74)85085-2.
- ↑ Pyykkö, P.; Desclaux, J. P. (1977). "Dirac–Fock one-centre calculations show (114)H4 to resemble PbH4". Nature. 266 (5600): 336–337. doi:10.1038/266336a0.
- ↑ CRC Handbook of Chemistry and Physics Online Edition.
- ↑ Visser, O.; Visscher, L.; Aerts, P. J. C.; Nieuwpoort, W. C. (1992). "Relativistic all-electron molecular Hartree-Fock-Dirac-(Breit) calculations on CH4, SiH4, GeH4, SnH4, PbH4". Theoretica Chimica Acta. 81 (6): 405–416. doi:10.1007/BF01134864.
- ↑ Malli, Gulzari L.; Siegert, Martin; Turner, David P. (2004). "Relativistic and electron correlation effects for molecules of heavy elements: Ab initio fully relativistic coupled-cluster calculations for PbH4". International Journal of Quantum Chemistry. 99 (6): 940–949. doi:10.1002/qua.20142.
- ↑ Krivtsun, V. M.; Kuritsyn, Y. A.; Snegirev, E. P. (1999). "Observation of IR absorption spectra of the unstable PbH4 molecule" (PDF). Opt. Spectrosc. 86: 686–691.
- ↑ Zou, Y; Jin, FX; Chen, ZJ; Qiu, DR; Yang, PY (2005). "Non-nascent hydrogen mechanism of plumbane generation". Guang pu xue yu guang pu fen xi = Guang pu. 25 (10): 1720–3. PMID 16395924.
- ↑ Wang, Xuefeng; Andrews, Lester (2003). "Infrared Spectra of Group 14 Hydrides in Solid Hydrogen: Experimental Observation of PbH4, Pb2H2, and Pb2H4". Journal of the American Chemical Society. 125 (21): 6581–6587. doi:10.1021/ja029862l. PMID 12785799.