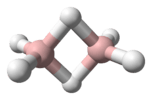Group 13 hydride
Group 13 hydrides are chemical compounds containing both hydrogen atoms and boron family atoms (elements of group 13: boron, aluminium, gallium, indium, thallium).
Trihydrides
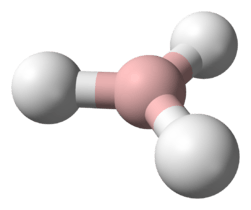
The simplest series has the chemical formula XH3, with X representing any of the boron family.
| Compound | Chemical formula | Model |
|---|---|---|
| (borane) | |  |
| (alane) | |  |
| (gallane) | | |
| (indigane) | | |
| (thallane) | |
The great variety of boranes show a huge covalent cluster chemistry, but the heavier group 13 hydrides do not. Despite their formulae, however, they tend to form polymers. Alane is a strong reducing agent with octahedrally coordinated aluminium atoms. Gallane is even harder to synthesise and decomposes to gallium and hydrogen at room temperature. Indigane and thallane are too unstable to exist for any significant time when not coordinated.[1]
They have a trigonal planar molecular geometry, contrasting with the trigonal pyramidal geometry of the pnictogen hydrides.
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 227–33. ISBN 0-08-037941-9.