Sodium hydride
 | |
 | |
| Identifiers | |
|---|---|
| 7646-69-7 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 23144 |
| ECHA InfoCard | 100.028.716 |
| EC Number | 231-587-3 |
| PubChem | 24758 |
| |
| |
| Properties | |
| NaH | |
| Molar mass | 23.99771 g/mol |
| Appearance | white or grey solid |
| Density | 1.396 g/cm3 |
| Melting point | 300 °C (572 °F; 573 K) decomposes |
| Reacts with water | |
| Solubility | insoluble in ammonia, benzene, CCl4, CS2 |
| Refractive index (nD) |
1.470 |
| Structure | |
| fcc (NaCl), cF8 | |
| Fm3m, No. 225 | |
| a = 498 pm | |
| Octahedral (Na+) Octahedral (H−) | |
| Thermochemistry | |
| 36.4 J/mol K | |
| Std molar entropy (S |
40 J·mol−1·K−1[1] |
| Std enthalpy of formation (ΔfH |
−56.4 kJ·mol−1[1] |
| Gibbs free energy (ΔfG˚) |
-33.5 kJ/mol |
| Hazards[2] | |
| Safety data sheet | External MSDS |
| GHS pictograms |  |
| GHS signal word | DANGER |
| H260 | |
| NFPA 704 | |
| Flash point | combustible |
| Related compounds | |
| Other cations |
Lithium hydride Potassium hydride |
| Related compounds |
Sodium borohydride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Sodium hydride is the chemical compound with the empirical formula NaH. This alkali metal hydride is primarily used as a strong, yet combustible base in organic synthesis. NaH is representative of the saline hydrides, meaning it is a salt-like hydride, composed of Na+ and H− ions, in contrast to the more molecular hydrides such as borane, methane, ammonia and water. It is an ionic material that is insoluble in organic solvents (although soluble in molten Na), consistent with the fact that H− remains an unknown anion in solution. Because of the insolubility of NaH, all reactions involving NaH occur at the surface of the solid.
Basic properties and structure
NaH is produced by the direct reaction of hydrogen and liquid sodium.[3] Pure NaH is colorless, although samples generally appear grey. NaH is ca. 40% denser than Na (0.968 g/cm3).
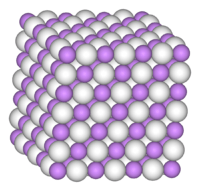
NaH, like LiH, KH, RbH, and CsH, adopts the NaCl crystal structure. In this motif, each Na+ ion is surrounded by six H− centers in an octahedral geometry. The ionic radii of H− (146 pm in NaH) and F− (133 pm) are comparable, as judged by the Na−H and Na−F distances.[4]
"Inverse sodium hydride"
A very unusual situation occurs in a compound dubbed "inverse sodium hydride", which contains Na− and H+ ions. Na− is an alkalide, and this compound differs from ordinary sodium hydride in having a much higher energy content due to the net displacement of two electrons from hydrogen to sodium. A derivative of this "inverse sodium hydride" arises in the presence of the base adamanzane. This molecule irreversibly encapsulates the H+ and shields it from interaction with the alkalide Na−.[5] Theoretical work has suggested that even an unprotected protonated tertiary amine complexed with the sodium alkalide might be metastable under certain solvent conditions, though the barrier to reaction would be small and finding a suitable solvent might be difficult.[6]
Applications in organic synthesis
As a strong base
NaH is a base of wide scope and utility in organic chemistry.[7] It is capable of deprotonating a range of even weak Brønsted acids to give the corresponding sodium derivatives. Typical "easy" substrates contain O-H, N-H, S-H bonds, including alcohols, phenols, pyrazoles, and thiols.
NaH most notably is employed to deprotonate carbon acids such as 1,3-dicarbonyls and analogues such as malonic esters. The resulting sodium derivatives can be alkylated. NaH is widely used to promote condensation reactions of carbonyl compounds via the Dieckmann condensation, Stobbe condensation, Darzens condensation, and Claisen condensation. Other carbon acids susceptible to deprotonation by NaH include sulfonium salts and DMSO. NaH is used to make sulfur ylides, which in turn are used to convert ketones into epoxides, as in the Johnson–Corey–Chaykovsky reaction.
As a reducing agent
NaH reduces certain main group compounds, but analogous reactivity is unknown in organic chemistry. Notably boron trifluoride reacts to give diborane and sodium fluoride:[3]
- 6 NaH + 2 BF3 → B2H6 + 6 NaF
Si-Si and S-S bonds in disilanes and disulfides are also reduced.
Drying agent
Because of its rapid and irreversible reaction with water, NaH can be used to dry some organic solvents. Other drying agents are far more widely used, such as calcium hydride.
Hydrogen storage
The use of sodium hydride has been proposed for hydrogen storage for use in fuel cell vehicles, the hydride being encased in plastic pellets which are crushed in the presence of water to release the hydrogen.[8]
Practical considerations
Sodium hydride is sold by many chemical suppliers usually as a mixture of 60% sodium hydride (w/w) in mineral oil. Such a dispersion is safer to handle and weigh than pure NaH. The compound is often used in this form but the pure grey solid can be prepared by rinsing the oil with pentane or THF, care being taken because the washings will contain traces of NaH that can ignite in air. Reactions involving NaH require an inert atmosphere, such as nitrogen or argon gas. Typically NaH is used as a suspension in THF, a solvent that resists deprotonation but solvates many organosodium compounds.
Safety
NaH can ignite in air, especially upon contact with water to release hydrogen, which is also flammable. Hydrolysis converts NaH into sodium hydroxide (NaOH), a caustic base. In practice, most sodium hydride is dispensed as a dispersion in oil, which can be safely handled in air.[9]
References
- 1 2 Zumdahl, Steven S. (2009). Chemical Principles 6th Ed. Houghton Mifflin Company. p. A23. ISBN 0-618-94690-X.
- ↑ Index no. 001-002-00-4 of Annex VI, Part 3, to Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJEU L353, 31.12.2008, pp 1–1355 at p 340.
- 1 2 Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ↑ Wells, A.F. (1984). Structural Inorganic Chemistry, Oxford: Clarendon Press
- ↑ Mikhail Y. Redko; et al. (2002). ""Inverse Sodium Hydride": A Crystalline Salt that Contains H+ and Na-". J. Am. Chem. Soc. 124 (21): 5928–5929. doi:10.1021/ja025655+.
- ↑ Agnieszka Sawicka; Piotr Skurski & Jack Simons (2003). "Inverse Sodium Hydride: A Theoretical Study" (PDF). J. Am. Chem. Soc. 125 (13): 3954–3958. doi:10.1021/ja021136v. PMID 12656631.
- ↑ Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289.
- ↑ J. Philip DiPietro; Edward G. Skolnik (October 1999). "Analysis of the Sodium Hydride-based Hydrogen Storage System being developed by PowerBall Technologies, LLC" (PDF). US Department of Energy, Office of Power Technologies. Retrieved 2009-09-01.
- ↑ MSDS 60% NaH in mineral oil