Gallium
 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| General properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, symbol | gallium, Ga | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation |
/ˈɡæliəm/ GAL-ee-əm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery blue | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Gallium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 31 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, block | group 13, p-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | post-transition metal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight (±) (Ar) | 69.723(1)[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d10 4s2 4p1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
per shell | 2, 8, 18, 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 302.9146 K (29.7646 °C, 85.5763 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 2673 K (2400 °C, 4352 °F)[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density near r.t. | 5.91 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid, at m.p. | 6.095 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 5.59 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 256 kJ/mol[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 25.86 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 3, 2, 1, −1, −2, −4, −5[3] (an amphoteric oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.81 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
1st: 578.8 kJ/mol 2nd: 1979.3 kJ/mol 3rd: 2963 kJ/mol (more) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 135 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 122±3 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 187 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure |
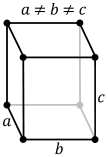 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 2740 m/s (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 18 µm/(m·K) (at 25 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 40.6 W/(m·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 270 nΩ·m (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 9.8 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.47 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 1.5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 56.8–68.7 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-55-3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | after Gallia (Latin for: France), homeland of the discoverer | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prediction | Dmitri Mendeleev (1871) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery and first isolation | Lecoq de Boisbaudran (1875) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes of gallium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Gallium is a chemical element with symbol Ga and atomic number 31. It is in group 13 of the periodic table, and thus has similarities to the other metals of the group, aluminium, indium, and thallium. Gallium does not occur as a free element in nature, but as gallium(III) compounds in trace amounts in zinc ores and in bauxite. Elemental gallium is a soft, silvery blue metal at standard temperature and pressure, a brittle solid at low temperatures, and a liquid at temperatures greater than 29.76 °C (85.57 °F) (slightly above room temperature). The melting point of gallium is used as a temperature reference point. The alloy galinstan (68.5% gallium, 21.5% indium, and 10% tin) has an even lower melting point of −19 °C (−2 °F), well below the freezing point of water.
Since its discovery in 1875, gallium has been used to make alloys with low melting points. It is also used in semiconductors as a dopant in semiconductor substrates.
Gallium is predominantly used in electronics. Gallium arsenide, the primary chemical compound of gallium in electronics, is used in microwave circuits, high-speed switching circuits, and infrared circuits. Semiconductive gallium nitride and indium gallium nitride produce blue and violet light-emitting diodes (LEDs) and diode lasers. Gallium is also used in the production of artificial gadolinium gallium garnet for jewelry.
Gallium has no known natural role in biology. Gallium(III) behaves in a similar manner to ferric salts in biological systems, and has been used in some medical applications, including pharmaceuticals and radiopharmaceuticals. Gallium is used in thermometers as a non-toxic and environmentally friendly alternative to mercury and can withstand higher temperatures than mercury.
Physical properties
Elemental gallium is not found in nature, but it is easily obtained by smelting. Very pure gallium metal has a silvery color and its solid metal fractures conchoidally like glass. Gallium liquid expands by 3.1% when it solidifies; therefore, it should not be stored in glass or metal containers because the container may rupture when the gallium changes state. Gallium shares the higher-density liquid state with a short list of other materials that includes water, silicon, germanium, antimony, bismuth, and plutonium.[4]
Gallium attacks most other metals by diffusing into the metal lattice. For example, it diffuses into the grain boundaries of aluminium-zinc alloys[5] and steel,[6] making them very brittle. Gallium easily alloys with many metals, and is used in small quantities in the plutonium-gallium alloy in the plutonium cores of nuclear bombs to stabilize the plutonium crystal structure.[7]
The melting point of gallium, at 302.9146 K (29.7646 °C, 85.5763 °F), is just above room temperature, and is approximately the same as the average summer daytime temperatures in Earth's mid-latitudes. This melting point (mp) is one of the formal temperature reference points in the International Temperature Scale of 1990 (ITS-90) established by the International Bureau of Weights and Measures (BIPM).[8][9][10] The triple point of gallium, 302.9166 K (29.7666 °C, 85.5799 °F), is used by the US National Institute of Standards and Technology (NIST) in preference to the melting point.[11]
The unique melting point of gallium allows it to melt in the human hand, and then refreeze if removed. The liquid metal has a strong tendency to supercool below its melting point/freezing point. Seeding with a crystal helps to initiate freezing. Gallium is one of the four metals (with caesium, rubidium, and mercury) that are known to be liquid at, or near, normal room temperature. Of the four, gallium is the only one that is neither highly reactive nor highly toxic and can therefore be used in metal-in-glass high-temperature thermometers. It is also notable for having one of the largest liquid ranges for a metal, and for having (unlike mercury) a low vapor pressure at high temperatures. Gallium's boiling point, 2673 K, is more than eight times higher than its melting point on the absolute scale, the greatest ratio between melting point and boiling point of any element.[12] Unlike mercury, liquid gallium metal wets glass and skin, along with most other materials (with the exceptions of quartz, graphite, and Teflon), making it mechanically more difficult to handle even though it is substantially less toxic and requires far fewer precautions. Gallium painted onto glass is a brilliant mirror.[13] For this reason as well as the metal contamination and freezing-expansion problems, samples of gallium metal are usually supplied in polyethylene packets within other containers.
| Property | a | b | c |
|---|---|---|---|
| α (~25 °C, µm/m) | 16 | 11 | 31 |
| ρ (29.7 °C, nΩ·m) | 543 | 174 | 81 |
| ρ (0 °C, nΩ·m) | 480 | 154 | 71.6 |
| ρ (77 K, nΩ·m) | 101 | 30.8 | 14.3 |
| ρ (4.2 K, pΩ·m) | 13.8 | 6.8 | 1.6 |
Gallium does not crystallize in any of the simple crystal structures. The stable phase under normal conditions is orthorhombic with 8 atoms in the conventional unit cell. Within a unit cell, each atom has only one nearest neighbor (at a distance of 244 pm). The remaining six unit cell neighbors are spaced 27, 30 and 39 pm farther away, and they are grouped in pairs with the same distance.[15] Many stable and metastable phases are found as function of temperature and pressure.[16]
The bonding between the two nearest neighbors is covalent; hence Ga2 dimers are seen as the fundamental building blocks of the crystal. This explains the low melting point relative to the neighbor elements, aluminium and indium. This structure is strikingly similar to that of iodine and forms because of interactions between the single 4p electrons of gallium atoms, further away from the nucleus than the 4s electrons and the [Ar]3d10 core. This phenomenon recurs with mercury with its "pseudo-noble-gas" [Xe]4f145d106s2 electron configuration, which is liquid at room temperature.[17] The 3d10 electrons do not shield the outer electrons very well from the nucleus and hence the first ionisation energy of gallium is greater than that of aluminium.[4]
The physical properties of gallium are highly anisotropic, i.e. have different values along the three major crystallographical axes a, b, and c (see table), producing a significant difference between the linear (α) and volume thermal expansion coefficients. The properties of gallium are strongly temperature-dependent, particularly near the melting point. For example, the coefficient of thermal expansion increases by several hundred percent upon melting.[14]
Chemical properties
Gallium is found primarily in the +3 oxidation state. The +1 oxidation is also found in some compounds. For example, the very stable GaCl2 contains both gallium(I) and gallium(III) and can be formulated as GaIGaIIICl4; in contrast, the monochloride is unstable above 0 °C disproportionating into elemental gallium and gallium(III) chloride. Compounds containing gallium-gallium bonds are true gallium(II) compounds; for example, GaS can be formulated Ga24+(S2−)2, and the dioxan complex Ga2Cl4(C4H8O2)2 contains a Ga–Ga bond.[18]
Chalcogen compounds
Gallium reacts with the chalcogens only at relatively high temperatures. At room temperature, gallium metal is not reactive with air and water because it forms a passive, protective oxide layer. At higher temperatures, however, it reacts with atmospheric oxygen to form gallium(III) oxide, Ga
2O
3.[19] Reducing Ga
2O
3 with elemental gallium in vacuum at 500 °C to 700 °C yields the dark brown gallium(I) oxide, Ga
2O.[20]:285 Ga
2O is a very strong reducing agent, capable of reducing H
2SO
4 to H
2S.[20]:207 It disproportionates at 800 °C back to gallium and Ga
2O
3.[21]
Gallium(III) sulfide, Ga
2S
3, has 3 possible crystal modifications.[21]:104 It can be made by the reaction of gallium with hydrogen sulfide (H
2S) at 950 °C.[20]:162 Alternatively, Ga(OH)
3 can be used at 747 °C:[22]
- 2 Ga(OH)
3 + 3 H
2S → Ga
2S
3 + 6 H
2O
Reacting a mixture of alkali metal carbonates and Ga
2O
3 with H
2S leads to the formation of thiogallates containing the [Ga
2S
4]2−
anion. Strong acids decompose these salts, releasing H
2S in the process.[21]:104–105 The mercury salt, HgGa
2S
4, can be used as a phosphor.[23]
Gallium also forms sulfides in lower oxidation states, such as gallium(II) sulfide and the green gallium(I) sulfide, the latter of which is produced from the former by heating to 1000 °C under a stream of nitrogen.[21]:94
The other binary chalcogenides, Ga
2Se
3 and Ga
2Te
3, have zincblende structure. They are all semiconductors but are easily hydrolysed and have limited utility.[21]:104
Aqueous chemistry
Strong acids dissolve gallium, forming gallium(III) salts such as Ga
2(SO
4)
3 (gallium sulfate) and Ga(NO
3)
3 (gallium nitrate). Aqueous solutions of gallium(III) salts contain the hydrated gallium ion, [Ga(H
2O)
6]3+
.[24]:1033 Gallium(III) hydroxide, Ga(OH)
3, may be precipitated from gallium(III) solutions by adding ammonia. Dehydrating Ga(OH)
3 at 100 °C produces gallium oxide hydroxide, GaO(OH).[20]:140–141
Alkaline hydroxide solutions dissolve gallium, forming gallate salts containing the Ga(OH)−
4 anion.[19][24]:1033[25] Gallium hydroxide, which is amphoteric, also dissolves in alkali to form gallate salts.[20]:141 Although earlier work suggested Ga(OH)3−
6 was another possible gallate anion,[26] it was not found in later work.[25]
Pnictogen compounds

Gallium reacts with ammonia at 1050 °C to form gallium nitride, GaN. Gallium also forms binary compounds with phosphorus, arsenic, and antimony: gallium phosphide (GaP), gallium arsenide (GaAs), and gallium antimonide (GaSb). These compounds have the same structure as ZnS, and have important semiconducting properties.[24]:1034 GaP, GaAs, and GaSb can be synthesized by the direct reaction of gallium with elemental phosphorus, arsenic, or antimony.[21]:99 They exhibit higher electrical conductivity than GaN.[21]:101 GaP can also be synthesized by reacting Ga
2O with phosphorus at low temperatures.[27]
Gallium forms ternary nitrides; for example:[21]:99
- Li
3Ga + N
2 → Li
3GaN
2
Similar compounds with phosphorus and arsenic are possible: Li
3GaP
2 and Li
3GaAs
2. These compounds are easily hydrolyzed by dilute acids and water.[21]:101
Halides
Gallium(III) oxide reacts with fluorinating agents such as HF or F
2 to form gallium(III) fluoride, GaF
3. It is an ionic compound strongly insoluble in water. However, it dissolves in hydrofluoric acid, in which it forms an adduct with water, GaF
3·3H
2O. Attempting to dehydrate this adduct forms GaF
2OH·nH
2O. The adduct reacts with ammonia to form GaF
3·3NH
3, which can then be heated to form anhydrous GaF
3.[20]:128–129
Gallium trichloride is formed by the reaction of gallium metal with chlorine gas.[19] Unlike the trifluoride, gallium(III) chloride exists as dimeric molecules, Ga
2Cl
6, with a melting point of 78 °C. Eqivalent compounds are formed with bromine and iodine, Ga
2Br
6 and Ga
2I
6.[20]:133
Like the other group 13 trihalides, gallium(III) halides are Lewis acids, reacting as halide acceptors with alkali metal halides to form salts containing GaX−
4 anions, where X is a halogen. They also react with alkyl halides to form carbocations and GaX−
4.[20]:136–137
When heated to a high temperature, gallium(III) halides react with elemental gallium to form the respective gallium(I) halides. For example, GaCl
3 reacts with Ga to form GaCl:
- 2 Ga + GaCl
3 ⇌ 3 GaCl (g)
At lower temperatures, the equilibrium shifts toward the left and GaCl disproportionates back to elemental gallium and GaCl
3. GaCl can also be produced by reacting Ga with HCl at 950 °C; the product can be condensed as a red solid.[24]:1036
Gallium(I) compounds can be stabilized by forming adducts with Lewis acids. For example:
- GaCl + AlCl
3 → Ga+
[AlCl
4]−
The so-called "gallium(II) halides", GaX
2, are actually adducts of gallium(I) halides with the respective gallium(III) halides, having the structure Ga+
[GaX
4]−
. For example:[19][24]:1036[28]
- GaCl + GaCl
3 → Ga+
[GaCl
4]−
Hydrogen compounds
Like aluminium, gallium also forms a hydride, GaH
3, known as gallane, which may be produced by reacting lithium gallanate (LiGaH
4) with gallium(III) chloride at −30 °C:[24]:1031
- 3 LiGaH
4 + GaCl
3 → 3 LiCl + 4 GaH
3
In the presence of dimethyl ether as solvent, GaH
3 polymerizes to (GaH
3)
n. If no solvent is used, the dimer Ga
2H
6 (digallane) is formed as a gas. Its structure is similar to diborane, having two hydrogen atoms bridging the two gallium centers,[24]:1031 unlike α-AlH
3 in which aluminium has a coordination number of 6.[24]:1008
Gallane is unstable above −10 °C, decomposing to elemental gallium and hydrogen.[29]
History

In 1871, the existence of gallium was first predicted by Russian chemist Dmitri Mendeleev, who named it "eka-aluminium" from its position in his periodic table. He also predicted several properties of eka-aluminium that correspond closely to the real properties of gallium, such as its density, melting point, oxide character and bonding in chloride.[30]
| Property | Mendeleev predictions | Actual properties |
|---|---|---|
| Atomic weight | ~68 | 69.723 |
| Density | 5.9 g/cm3 | 5.904 g/cm3 |
| Melting point | Low | 29.767 °C |
| Formula of oxide | M2O3 | Ga2O3 |
| Density of oxide | 5.5 g/cm3 | 5.88 g/cm3 |
| Nature of hydroxide | amphoteric | amphoteric |
Mendeleev further predicted that eka-aluminium would be discovered by means of the spectroscope, and that metallic eka-aluminium would dissolve slowly in both acids and alkalis and would not react with air. He also predicted that M2O3 would dissolve in acids to give MX3 salts, that eka-aluminium salts would form basic salts, that eka-aluminium sulfate should form alums, and that anhydrous MCl3 should have a greater volatility than ZnCl2: all of these predictions turned out to be true.[31]
Gallium was discovered using spectroscopy by French chemist Paul Emile Lecoq de Boisbaudran in 1875 from its characteristic spectrum (two violet lines) in a sample of sphalerite.[32] Later that year, Lecoq obtained the free metal by electrolysis of the hydroxide in potassium hydroxide solution. He named the element "gallia", from Latin Gallia meaning Gaul, after his native land of France. It was later claimed that, in one of those multilingual puns so beloved by men of science in the 19th century, he had also named gallium after himself: "Le coq" is French for "the rooster" and the Latin word for "rooster" is "gallus". In an 1877 article, Lecoq denied this conjecture.[33] Originally, de Boisbaudran determined the density of gallium as 4.7 g/cm3, the only property that failed to match Mendeleev's predictions; Mendeleev then wrote to him and suggested that he should remeasure the density, and de Boisbaudran then obtained the correct value of 5.9 g/cm3, that Mendeleev had predicted almost exactly.[31]
From its discovery in 1875 until the era of semiconductors, the primary uses of gallium were high-temperature thermometrics and metal alloys with unusual properties of stability or ease of melting (some such being liquid at room temperature). The development of gallium arsenide as a direct band gap semiconductor in the 1960s ushered in the most important stage in the applications of gallium.[13]
Occurrence
Gallium does not exist as a free element in the Earth's crust, and the few high-content minerals, such as gallite (CuGaS2), are too rare to serve as a primary source. The abundance in the Earth's crust is approximately 16.9 ppm.[34] Gallium is found and extracted as a trace component in bauxite and to a small extent from sphalerite. The amount extracted from coal, diaspore and germanite, in which gallium is also present, is negligible. The United States Geological Survey (USGS) estimates that gallium reserves exceed 1 million tonnes, based on 50 ppm by weight concentration in known reserves of bauxite and zinc ores.[35][36] Some coal flue dusts contain small quantities of gallium, typically less than 1% by weight.[37][38][39][40]
Production

The primary source of gallium is a byproduct of the production of aluminium and zinc, while the sphalerite from zinc production is a minor source. Most gallium is extracted from the crude aluminium hydroxide solution used in the Bayer process for producing alumina and aluminium. A mercury-cell electrolysis and hydrolysis of the amalgam with sodium hydroxide leads to sodium gallate. Electrolysis then gives gallium metal. For semiconductor use, it is further purified with zone melting or single-crystal extraction from a melt (Czochralski process). Purities of 99.9999% are routinely achieved and commercially available.[41]
In 1986, gallium production was estimated at 40 tons.[42] In 2007, production of gallium was 184 tonnes, of which less than 100 tonnes was from mining, with the rest from scrap recycling.[35] By 2012, world production of gallium was an estimated 273 metric tons.[43] In January 2013, the market price of gallium lay at 580 USD/kg, while by autumn that year, it had dropped to around 280 USD/kg, or just under 9 USD per ounce.[44]
Applications
Semiconductor applications dominate the commercial demand for gallium, accounting for 98% of the total. The next major application is for gadolinium gallium garnets.[42]
Semiconductors

Extremely high-purity (99.9999+%) gallium is commercially available to serve the semiconductor industry. Gallium arsenide (GaAs) and gallium nitride (GaN) used in electronic components represented about 98% of the gallium consumption in the United States in 2007. About 66% of semiconductor gallium is used in the U.S. in integrated circuits (mostly gallium arsenide), such as the manufacture of ultra-high speed logic chips and MESFETs for low-noise microwave preamplifiers in cell phones. About 20% of this gallium is used in optoelectronics.[35] Worldwide, gallium arsenide makes up 95% of the annual global gallium consumption.[41]
Gallium arsenide is used in a variety of optoelectronic infrared devices. Aluminium gallium arsenide (AlGaAs) is used in high-power infrared laser diodes. The semiconductors gallium nitride and indium gallium nitride are used in blue and violet optoelectronic devices, mostly laser diodes and light-emitting diodes. For example, gallium nitride 405 nm diode lasers are used as a violet light source for higher-density Blu-ray Disc compact data disc drives.[45]
Multijunction photovoltaic cells, developed for satellite power applications, are made by molecular beam epitaxy or metalorganic vapour phase epitaxy of thin films of gallium arsenide, indium gallium phosphide, or indium gallium arsenide.The Mars Exploration Rovers and several satellites use triple junction gallium arsenide on germanium cells.[46] Gallium is also a component in photovoltaic compounds (such as copper indium gallium selenium sulfide Cu(In,Ga)(Se,S)2) used in solar panels as a cost-efficient alternative to crystalline silicon.[47]
Galinstan and other alloys

Gallium readily alloys with most metals, and is used as an ingredient in low-melting alloys. The nearly eutectic alloy of gallium, indium, and tin is a room temperature liquid used in medical thermometers. This alloy, with the trade-name Galinstan (with the "-stan" referring to the tin, stannum in Latin), has a low freezing point of −19 °C (−2.2 °F).[48] It has been suggested that this family of alloys could also be used to cool computer chips in place of water.[49] Gallium alloys have been evaluated as substitutes for mercury dental amalgams, but these materials have yet to see wide acceptance.
Because gallium wets glass or porcelain, gallium can be used to create brilliant mirrors. When the wetting action of gallium-alloys is not desired (as in Galinstan glass thermometers), the glass must be protected with a transparent layer of gallium(III) oxide.[50]
The plutonium used in nuclear weapon pits is stabilized in the δ phase and made machinable by alloying with gallium.[51]
Biomedical applications
Although gallium has no natural function in biology, gallium ions interact with processes in the body in a manner similar to iron(III). Because these processes include inflammation, a marker for many disease states, several gallium salts are used (or are in development) as pharmaceuticals and radiopharmaceuticals in medicine. When gallium ions are mistakenly taken up in place of iron(III) by bacteria such as Pseudomonas, the ions interfere with respiration, and the bacteria die. This happens because iron is redox-active, allowing the transfer of electrons during respiration, while gallium is redox-inactive.[52][53]
Gallium nitrate (brand name Ganite) has been used as an intravenous pharmaceutical to treat hypercalcemia associated with tumor metastasis to bones. Gallium is thought to interfere with osteoclast function, and the therapy may be effective when other treatments have failed.[54]
Gallium maltolate, an oral, highly absorbable form of gallium(III) ion, is an anti-proliferative to pathologically proliferating cells, particularly cancer cells and some bacteria that accept it in place of ferric iron (Fe3+). Researchers are conducting clinical and preclinical trials on this compound as a potential treatment for a number of cancers, infectious diseases, and inflammatory diseases.[55]
A complex amine-phenol Ga(III) compound MR045 is selectively toxic to parasites resistant to chloroquine, a common drug against malaria. Both the Ga(III) complex and chloroquine act by inhibiting crystallization of hemozoin, a disposal product formed from the digestion of blood by the parasites.[56][57]
Radiogallium salts
Gallium-67 salts such as gallium citrate and gallium nitrate are used as radiopharmaceutical agents in the nuclear medicine imaging known as gallium scan. The radioactive isotope 67Ga is used, and the compound or salt of gallium is unimportant. The body handles Ga3+ in many ways as though it were Fe3+, and the ion is bound (and concentrates) in areas of inflammation, such as infection, and in areas of rapid cell division. This allows such sites to be imaged by nuclear scan techniques.[58]
Gallium-68, a positron emitter with a half-life of 68 min, is now used as a diagnostic radionuclide in PET-CT when linked to pharmaceutical preparations such as DOTATOC, a somatostatin analogue used for neuroendocrine tumors investigation, and DOTA-TATE, a newer one, used for neuroendocrine metastasis and lung neuroendocrine cancer, such as certain types of microcytoma. Gallium-68's preparation as a pharmaceutical is chemical and the radionuclide is extracted by elution from germanium-68, a synthetic radioisotope of germanium, in gallium-68 generators.[59]
Other uses
Gallium is used for neutrino detection. Possibly the largest amount of pure gallium ever collected in a single spot is the Gallium-Germanium Neutrino Telescope used by the SAGE experiment at the Baksan Neutrino Observatory in Russia. This detector contains 55–57 tonnes of liquid gallium.[60] Another experiment was the GALLEX neutrino detector operated in the early 1990s in an Italian mountain tunnel. The detector contained 12.2 tons of watered gallium-71. Solar neutrinos caused a few atoms of 71Ga to become radioactive 71Ge, which were detected. This experiment showed that the solar neutrino flux is 40% less than theory predicted. This deficit was not explained until better solar neutrino detectors and theories were constructed (see SNO).[61]
Gallium is also used as a liquid metal ion source for a focused ion beam. For example, a focused gallium-ion beam was used to create the world's smallest book, Teeny Ted from Turnip Town.[62] Another use of gallium is as an additive in glide wax for skiis, and other low-friction surface materials.[63]
A well-known practical joke among chemists is to fashion gallium spoons and use them to serve tea to unsuspecting guests, since gallium has a similar appearance to its lighter homolog aluminium. The spoons then melt in the hot tea.[64]
Precautions
Metallic gallium is not toxic. However, exposure to gallium halide complexes can result in acute toxicity.[65] The Ga3+ ion of soluble gallium salts tends to form the insoluble hydroxide when injected in large doses; precipitation of this hydroxide resulted in renal toxicity in animals. In lower doses, soluble gallium is tolerated well and does not accumulate as a poison, instead being excreted mostly through urine. Excretion of gallium occurs in two phases: the first phase has a biological half-life of one hour while the second has a biological half-life of twenty-five hours.[58]
References
- ↑ Standard Atomic Weights 2013. Commission on Isotopic Abundances and Atomic Weights
- 1 2 Zhang Y; Evans JRG; Zhang S (2011). "Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks". J. Chem. Eng. Data. 56 (2): 328–337. doi:10.1021/je1011086.
- ↑ Hofmann, Patrick (1997). Colture. Ein Programm zur interaktiven Visualisierung von Festkörperstrukturen sowie Synthese, Struktur und Eigenschaften von binären und ternären Alkali- und Erdalkalimetallgalliden (PDF) (in German). PhD Thesis, ETH Zurich. p. 72. doi:10.3929/ethz-a-001859893. ISBN 3728125970.
- 1 2 Greenwood and Earnshaw, p. 222
- ↑ Tsai, W. L; Hwu, Y.; Chen, C.H.; Chang, L.W.; Je, J.H.; Lin, H.M.; Margaritondo, G. (2003). "Grain boundary imaging, gallium diffusion and the fracture behavior of Al–Zn Alloy – An in situ study". Nuclear Instruments and Methods in Physics Research Section B. 199: 457. Bibcode:2003NIMPB.199..457T. doi:10.1016/S0168-583X(02)01533-1.
- ↑ Vigilante, G. N.; Trolano, E.; Mossey, C. (June 1999). "Liquid Metal Embrittlement of ASTM A723 Gun Steel by Indium and Gallium". Defense Technical Information Center. Retrieved 2009-07-07.
- ↑ Sublette, Cary (2001-09-09). "Section 6.2.2.1". Nuclear Weapons FAQ. Retrieved 2008-01-24.
- ↑ Preston–Thomas, H. (1990). "The International Temperature Scale of 1990 (ITS-90)" (PDF). Metrologia. 27: 3–10. Bibcode:1990Metro..27....3P. doi:10.1088/0026-1394/27/1/002.
- ↑ "ITS-90 documents at Bureau International de Poids et Mesures".
- ↑ Magnum, B.W.; Furukawa, G.T. (August 1990). "Guidelines for Realizing the International Temperature Scale of 1990 (ITS-90)" (PDF). National Institute of Standards and Technology. NIST TN 1265.
- ↑ Strouse, Gregory F. (1999). "NIST realization of the gallium triple point". National Institute of Standards and Technology. Proc. TEMPMEKO 1999 1 (1999): 147-152. Retrieved 2016-10-30.
- ↑ Greenwood and Earnshaw, p. 224
- 1 2 Greenwood and Earnshaw, p. 221
- 1 2 Rosebury, Fred (1992). Handbook of Electron Tube and Vacuum Techniques. Springer. p. 26. ISBN 978-1-56396-121-2.
- ↑ M. Bernascino; et al. (1995). "Ab initio calculations of structural and electronic properties of gallium solid-state phases". Phys. Rev. B. 52: 9988. Bibcode:1995PhRvB..52.9988B. doi:10.1103/PhysRevB.52.9988.
- ↑ "Phase Diagrams of the Elements", David A. Young, UCRL-51902 "Prepared for the U.S. Energy Research & Development Administration under contract No. W-7405-Eng-48". (1975)
- ↑ Greenwood and Earnshaw, p. 223
- ↑ Greenwood and Earnshaw, p. 240
- 1 2 3 4 Eagleson, Mary, ed. (1994). Concise encyclopedia chemistry. Walter de Gruyter. p. 438. ISBN 3-11-011451-8.
- 1 2 3 4 5 6 7 8 Downs, Anthony John (1993). Chemistry of aluminium, gallium, indium, and thallium. Springer. ISBN 0-7514-0103-X.
- 1 2 3 4 5 6 7 8 9 Greenwood, N. N. (1962). Harry Julius Emeléus; Alan G. Sharpe, eds. Advances in inorganic chemistry and radiochemistry. 5. Academic Press. pp. 94–95. ISBN 0-12-023605-2.
- ↑ Madelung, Otfried (2004). Semiconductors: data handbook (3rd ed.). Birkhäuser. pp. 276–277. ISBN 3-540-40488-0.
- ↑ Krausbauer, L.; Nitsche, R.; Wild, P. (1965). "Mercury gallium sulfide, HgGa
2S
4, a new phosphor". Physica. 31 (1): 113–121. Bibcode:1965Phy....31..113K. doi:10.1016/0031-8914(65)90110-2. - 1 2 3 4 5 6 7 8 Wiberg, Egon; Wiberg, Nils; Holleman, Arnold Frederick (2001). Inorganic chemistry. Academic Press. ISBN 0-12-352651-5.
- 1 2 Sipos, P. L.; Megyes, T. N.; Berkesi, O. (2008). "The Structure of Gallium in Strongly Alkaline, Highly Concentrated Gallate Solutions—a Raman and 71
Ga
-NMR Spectroscopic Study". J Solution Chem. 37 (10): 1411–1418. doi:10.1007/s10953-008-9314-y. - ↑ Hampson, N. A. (1971). Harold Reginald Thirsk, ed. Electrochemistry—Volume 3: Specialist periodical report. Great Britain: Royal Society of Chemistry. p. 71. ISBN 0-85186-027-3.
- ↑ Michelle Davidson (2006). Inorganic Chemistry. Lotus Press. p. 90. ISBN 81-89093-39-8.
- ↑ Arora, Amit (2005). Text Book Of Inorganic Chemistry. Discovery Publishing House. pp. 389–399. ISBN 81-8356-013-X.
- ↑ Downs, Anthony J.; Pulham, Colin R. (1994). Sykes, A. G., ed. Advances in Inorganic Chemistry. 41. Academic Press. pp. 198–199. ISBN 0-12-023641-9.
- ↑ Ball, Philip (2002). The Ingredients: A Guided Tour of the Elements. Oxford University Press. p. 105. ISBN 0-19-284100-9.
- 1 2 3 Greenwood and Earnshaw, p. 217
- ↑ de Boisbaudran, Lecoq (1835–1965). "Caractères chimiques et spectroscopiques d'un nouveau métal, le gallium, découvert dans une blende de la mine de Pierrefitte, vallée d'Argelès (Pyrénées)". Comptes rendus. 81: 493. Retrieved 2008-09-23.
- ↑ Weeks, Mary Elvira (1932). "The discovery of the elements. XIII. Some elements predicted by Mendeleeff". Journal of Chemical Education. 9 (9): 1605–1619. Bibcode:1932JChEd...9.1605W. doi:10.1021/ed009p1605.
- ↑ Burton, J. D.; Culkin, F.; Riley, J. P. (2007). "The abundances of gallium and germanium in terrestrial materials". Geochimica et Cosmochimica Acta. 16: 151. Bibcode:1959GeCoA..16..151B. doi:10.1016/0016-7037(59)90052-3.
- 1 2 3 Kramer, Deborah A. "Mineral Commodity Summary 2006: Gallium" (PDF). United States Geological Survey. Retrieved 2008-11-20.
- ↑ Kramer, Deborah A. "Mineral Yearbook 2006: Gallium" (PDF). United States Geological Survey. Retrieved 2008-11-20.
- ↑ Xiao-quan, Shan; Wen, Wang & Bei, Wen (1992). "Determination of gallium in coal and coal fly ash by electrothermal atomic absorption spectrometry using slurry sampling and nickel chemical modification". Journal of Analytical Atomic Spectrometry. 7 (5): 761. doi:10.1039/JA9920700761.
- ↑ "Gallium in West Virginia Coals". West Virginia Geological and Economic Survey. 2002-03-02.
- ↑ Font, O; Querol, Xavier; Juan, Roberto; Casado, Raquel; Ruiz, Carmen R.; López-Soler, Ángel; Coca, Pilar; Peña, Francisco García (2007). "Recovery of gallium and vanadium from gasification fly ash". Journal of Hazardous Materials. 139 (3): 413–23. doi:10.1016/j.jhazmat.2006.02.041. PMID 16600480.
- ↑ Headlee, A. J. W. & Hunter, Richard G. (1953). "Elements in Coal Ash and Their Industrial Significance". Industrial and Engineering Chemistry. 45 (3): 548. doi:10.1021/ie50519a028.
- 1 2 Moskalyk, R. R. (2003). "Gallium: the backbone of the electronics industry". Minerals Engineering. 16 (10): 921. doi:10.1016/j.mineng.2003.08.003.
- 1 2 Greber, J. F. (2012) "Gallium and Gallium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, doi:10.1002/14356007.a12_163.
- ↑ Gallium report – U.S. Geological Survey, Mineral Commodity Summaries, January 2013
- ↑ USGS Minerals Commodity Surveys: "Gallium" (2013 ed)
- ↑ Coleman, James J; Jagadish, Chennupati; Catrina Bryce, A (2012-05-02). Advances in Semiconductor Lasers. pp. 150–151. ISBN 978-0-12-391066-0.
- ↑ Crisp, D.; Pathare, A.; Ewell, R. C. (2004). "The performance of gallium arsenide/germanium solar cells at the Martian surface". Acta Astronautica. 54 (2): 83. Bibcode:2004AcAau..54...83C. doi:10.1016/S0094-5765(02)00287-4.
- ↑ Alberts, V.; Titus J.; Birkmire R. W. (2003). "Material and device properties of single-phase Cu(In,Ga)(Se,S)2 alloys prepared by selenization/sulfurization of metallic alloys". Thin Solid Films. 451–452: 207. Bibcode:2004TSF...451..207A. doi:10.1016/j.tsf.2003.10.092.
- ↑ Surmann, P; Zeyat, H (Nov 2005). "Voltammetric analysis using a self-renewable non-mercury electrode". Analytical and Bioanalytical Chemistry. 383 (6): 1009–13. doi:10.1007/s00216-005-0069-7. ISSN 1618-2642. PMID 16228199.
- ↑ Knight, Will (2005-05-05). "Hot chips chilled with liquid metal". Retrieved 2008-11-20.
- ↑ United States. Office of Naval Research. Committee on the Basic Properties of Liquid Metals,U.S. Atomic Energy Commission (1954). Liquid-metals handbook. U.S. Govt. Print. Off. p. 128.
- ↑ Besmann, Theodore M. (2005). "Thermochemical Behavior of Gallium in Weapons-Material-Derived Mixed-Oxide Light Water Reactor (LWR) Fuel". Journal of the American Ceramic Society. 81 (12): 3071. doi:10.1111/j.1151-2916.1998.tb02740.x.
- ↑ "A Trojan-horse strategy selected to fight bacteria". INFOniac.com. 2007-03-16. Retrieved 2008-11-20.
- ↑ Smith, Michael (2007-03-16). "Gallium May Have Antibiotic-Like Properties". MedPage Today. Retrieved 2008-11-20.
- ↑ "gallium nitrate". Retrieved 2009-07-07.
- ↑ Bernstein, L. R.; Tanner, T.; Godfrey, C. & Noll, B. (2000). "Chemistry and Pharmacokinetics of Gallium Maltolate, a Compound With High Oral Gallium Bioavailability". Metal-Based Drugs. 7 (1): 33–47. doi:10.1155/MBD.2000.33. PMC 2365198
 . PMID 18475921.
. PMID 18475921. - ↑ Goldberg D. E.; Sharma V.; Oksman A.; Gluzman I. Y.; Wellems T. E.; Piwnica-Worms D. (1997). "Probing the chloroquine resistance locus of Plasmodium falciparum with a novel class of multidentate metal(III) coordination complexes". J. Biol. Chem. 272 (10): 6567–72. doi:10.1074/jbc.272.10.6567. PMID 9045684.
- ↑ Biot, Christophe; Dive, Daniel (2010). "Bioorganometallic Chemistry and Malaria". Medicinal Organometallic Chemistry. Topics in Organometallic Chemistry. 32. p. 155. doi:10.1007/978-3-642-13185-1_7. ISBN 978-3-642-13184-4.
- 1 2 Nordberg, Gunnar F.; Fowler, Bruce A.; Nordberg, Monica (7 August 2014). Handbook on the Toxicology of Metals (4th ed.). Academic Press. pp. 788–90. ISBN 978-0-12-397339-9.
- ↑ Banerjee, Sangeeta Ray; Pomper, Martin G. (June 2013). "Clinical Applications of Gallium-68". Appl. Radiat. Isot. June (0): 2–13. doi:10.1016/j.apradiso.2013.01.039. PMC 3664132
 .
. - ↑ "Russian American Gallium Experiment". 2001-10-19. Retrieved 2009-06-24.
- ↑ "Neutrino Detectors Experiments: GALLEX". 1999-06-26. Retrieved 2008-11-20.
- ↑ "Nano lab produces world's smallest book". Simon Fraser University. 11 April 2007. Retrieved 31 January 2013.
- ↑ US 5069803, Sugimura, Kentaro; Shoji Hasimoto & Takayuki Ono, "Use of a synthetic resin composition containing gallium particles in the glide surfacing material of skis and other applications", issued 1995
- ↑ Sam Kean (2010). The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the Elements. Boston: Little, Brown and Company. ISBN 0-316-05164-0.
- ↑ Ivanoff, C. S.; Ivanoff, A. E.; Hottel, T. L. (February 2012). "Gallium poisoning: a rare case report.". Food Chem Toxicol. 50 (2): 212–5. doi:10.1016/j.fct.2011.10.041. PMID 22024274.
Bibliography
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
External links
| Wikimedia Commons has media related to Gallium. |
| Wikisource has the text of the 1911 Encyclopædia Britannica article Gallium. |
- Gallium at The Periodic Table of Videos (University of Nottingham)
- Safety data sheet at acialloys.com
- High-resolution photographs of molten gallium, gallium crystals and gallium ingots under Creative Commons licence
- www.lenntech.com – textbook information regarding gallium
- Environmental effects of gallium
- Price development of gallium 1959–1998
- Gallium : A Smart Metal United States Geological Survey
- Technology produces hydrogen by adding water to an alloy of aluminum and gallium
- Thermal conductivity
- Physical and thermodynamical properties of liquid gallium (doc pdf)
| Periodic table (Large cells) | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||||||||||||||||
| 1 | H | He | |||||||||||||||||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | |||||||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||||||||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |||||||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |||||||||||||||
| 6 | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | |
| 7 | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
|
| |||||||||||||||||||||||||||||||||