Darmstadtium
| General properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, symbol | darmstadtium, Ds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation |
darm-SHTAHT-ee-əm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Darmstadtium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 110 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, block | group 10, d-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | unknown, but probably a transition metal | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight (Ar) | [281] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration |
[Rn] 5f14 6d8 7s2 (predicted)[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
per shell | 2, 8, 18, 32, 32, 16, 2 (predicted)[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid (predicted)[3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density near r.t. | 34.8 g/cm3 (predicted)[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 8, 6, 4, 2, 0 (predicted)[2][4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
1st: 955.2 kJ/mol 2nd: 1891.1 kJ/mol 3rd: 3029.6 kJ/mol (more) (all estimated)[2] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 132 pm (predicted)[2][4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 128 pm (estimated)[5] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure |
body-centered cubic (bcc) 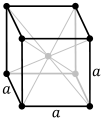
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 54083-77-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | after Darmstadt, Germany, where it was discovered | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Gesellschaft für Schwerionenforschung (1994) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes of darmstadtium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Darmstadtium is a chemical element with symbol Ds and atomic number 110. It is an extremely radioactive synthetic element. The most stable known isotope, darmstadtium-281, has a half-life of approximately 10 seconds.[6] Darmstadtium was first created in 1994 by the GSI Helmholtz Centre for Heavy Ion Research near the city of Darmstadt, Germany, after which it was named.
In the periodic table, it is a d-block transactinide element. It is a member of the 7th period and is placed in the group 10 elements, although no chemical experiments have yet been carried out to confirm that it behaves as the heavier homologue to platinum in group 10 as the eighth member of the 6d series of transition metals. Darmstadtium is calculated to have similar properties to its lighter homologues, nickel, palladium, and platinum.
History

Discovery
Darmstadtium was first created on November 9, 1994, at the Institute for Heavy Ion Research (Gesellschaft für Schwerionenforschung, GSI) in Darmstadt, Germany, by Peter Armbruster and Gottfried Münzenberg, under the direction of Sigurd Hofmann. The team bombarded a lead-208 target with accelerated nuclei of nickel-62 in a heavy ion accelerator and detected a single atom of the isotope darmstadtium-269:[7]
- 208
82Pb + 62
28Ni → 269
110Ds + 1
0n
In the same series of experiments, the same team also carried out the reaction using heavier nickel-64 ions. During two runs, 9 atoms of 271Ds were convincingly detected by correlation with known daughter decay properties:[8]
- 208
82Pb + 64
28Ni → 271
110Ds + 1
0n
Prior to this, there had been failed synthesis attempts in 1986–7 at the Joint Institute for Nuclear Research in Dubna (then in the Soviet Union) and in 1990 at the GSI.[9] The IUPAC/IUPAP Joint Working Party (JWP) recognised the GSI team as discoverers in their 2001 report.[10]
Naming
Using Mendeleev's nomenclature for unnamed and undiscovered elements, darmstadtium should be known as eka-platinum. In 1979, IUPAC published recommendations according to which the element was to be called ununnilium (with the corresponding symbol of Uun),[11] a systematic element name as a placeholder, until the element was discovered (and the discovery then confirmed) and a permanent name was decided on. Although widely used in the chemical community on all levels, from chemistry classrooms to advanced textbooks, the recommendations were mostly ignored among scientists in the field, who called it "element 110", with the symbol of (110) or even simply 110.[2]
In 1996, the Russian team proposed the name becquerelium after Henri Becquerel.[12] The American team in 1997 proposed the name hahnium[13] after Otto Hahn (previously this name had been used for element 105).
The name darmstadtium (Ds) was suggested by the GSI team in honor of the city of Darmstadt, where the element was discovered.[14][15] The GSI team originally also considered naming the element wixhausium, after the suburb of Darmstadt known as Wixhausen where the element was discovered, but eventually decided on darmstadtium.[16] The new name was officially recommended by IUPAC on August 16, 2003.[14]
Isotopes
- For a detailed list of information on the discovery of each individual darmstadtium isotope, see isotopes of darmstadtium.
Darmstadtium has no stable or naturally-occurring isotopes. Several radioactive isotopes have been synthesized in the laboratory, either by fusing two atoms or by observing the decay of heavier elements. Nine different isotopes of darmstadtium have been reported with atomic masses 267, 269–271, 273, 277, and 279–281, although darmstadtium-267 and darmstadtium-280 are unconfirmed. Two darmstadtium isotopes, darmstadtium-270 and darmstadtium-271, have known metastable states. Most of these decay predominantly through alpha decay, but some undergo spontaneous fission.[17]
Stability and half-lives
All darmstadtium isotopes are extremely unstable and radioactive; in general, the heavier isotopes are more stable than the lighter. The most stable known darmstadtium isotope, 281Ds, is also the heaviest known darmstadtium isotope; it has a half-life of 11 seconds. The isotope 279Ds has a half-life of 0.18 seconds respectively. The remaining six isotopes and two metastable states have half-lives between 1 microsecond and 70 milliseconds.[17] Some unknown isotopes in this region, such as 272Ds and 274–276Ds, are predicted to also have rather long half-lives of a few seconds.[17][18] Before its discovery, 277Ds was predicted to have a half-life of around 5 seconds, but it has since been found to have a half-life of only 5.7 milliseconds.[17] Likewise, while 280Ds was originally predicted to have a half-life of about 11 seconds, more recent work that may have detected it as the electron-capture daughter of 280Rg, have found for it a half-life of much less than a second.[19]
| Isotope | Half-life [17][18] | Decay mode[17][18] | Discovery year | Reaction |
|---|---|---|---|---|
| 267Ds ? | 2.8 μs | α | 1994 | 209Bi(59Co,n)[20] |
| 268Ds | 100? μs | α ? | unknown | — |
| 269Ds | 179 μs | α | 1994 | 208Pb(62Ni,n)[7] |
| 270Ds | 100 μs | α, SF | 2000 | 207Pb(64Ni,n)[21] |
| 270mDs | 6.0 ms | α, IT | 2000 | 207Pb(64Ni,n)[21] |
| 271Ds | 1.63 ms | α | 1994 | 208Pb(64Ni,n)[8] |
| 271mDs | 69 ms | α | 1994 | 208Pb(64Ni,n)[8] |
| 272Ds | 1? s | SF ? | unknown | — |
| 273Ds | 170 μs | α | 1996 | 244Pu(34S,5n)[22] |
| 274Ds | 2? s | α, SF ? | unknown | — |
| 275Ds | 100? μs[23] | α ? | unknown | — |
| 276Ds | 2.3? ms[23] | α ? | unknown | — |
| 277Ds | 5.7 ms | α | 2010 | 285Fl(—,2α)[24] |
| 278Ds | 10? s | α, SF ? | unknown | — |
| 279Ds | 0.18 s | SF, α | 2002 | 291Lv(—,3α)[25] |
| 280Ds ? | << 1 s | SF | 2015 | 280Rg( e− , ν e)?[19] |
| 281Ds | 9.6 s | SF, α | 1999 | 289Fl(—,2α)[6] |
The undiscovered isotope 284Ds has been predicted to be the most stable towards beta decay;[26] however, no known darmstadtium isotope has been observed to undergo beta decay.[17] Theoretical calculation in a quantum tunneling model reproduces the experimental alpha decay half-life data for the known darmstadtium isotopes.[27][28] It also predicts that the undiscovered isotope 294Ds, which has a magic number of neutrons (184),[2] would have an alpha decay half-life on the order of 311 years: exactly the same approach predicts a ~3500-year alpha half-life for the non-magic 293Ds isotope, however.[29][30]
Predicted properties
Chemical
Darmstadtium is the eighth member of the 6d series of transition metals. Since copernicium (element 112) has been shown to be a transition metal, it is expected that all the elements from 104 to 112 would form a fourth transition metal series, with darmstadtium as part of the platinum group metals[15] and a noble metal.[2] Calculations on its ionization potentials and atomic and ionic radii are similar to that of its lighter homologue platinum, thus implying that darmstadtium's basic properties will resemble those of the other group 10 elements, nickel, palladium, and platinum.[2]
Prediction of the probable chemical properties of darmstadtium has not received much attention recently. Darmstadtium is expected to be a noble metal. Based on the most stable oxidation states of the lighter group 10 elements, the most stable oxidation states of darmstadtium are predicted to be the +6, +4, and +2 states; however, the neutral state is predicted to be the most stable in aqueous solutions. In comparison, only palladium and platinum are known to show the maximum oxidation state in the group, +6, while the most stable states are +4 and +2 for both nickel and palladium. It is further expected that the maximum oxidation states of elements from bohrium (element 107) to darmstadtium (element 110) may be stable in the gas phase but not in aqueous solution.[2] Darmstadtium hexafluoride (DsF6) is predicted to have very similar properties to its lighter homologue platinum hexafluoride (PtF6), having very similar electronic structures and ionization potentials.[2][31][32] It is also expected to have the same octahedral molecular geometry as PtF6.[33] Other predicted darmstadtium compounds are darmstadtium carbide (DsC) and darmstadtium tetrachloride (DsCl4), both of which are expected to behave like their lighter homologues.[33]
Physical and atomic
Darmstadtium is expected to be a solid under normal conditions and to crystallize in the body-centered cubic structure, unlike its lighter congeners which crystallize in the face-centered cubic structure, because it is expected to have different electron charge densities from them.[3] It should be a very heavy metal with a density of around 34.8 g/cm3. In comparison, the densest known element that has had its density measured, osmium, has a density of only 22.61 g/cm3.[2] This results from darmstadtium's high atomic weight, the lanthanide and actinide contractions, and relativistic effects, although production of enough darmstadtium to measure this quantity would be impractical, and the sample would quickly decay.[2]
The outer electron configuration of darmstadtium is calculated to be 6d87s2, which obeys the Aufbau principle and does not follow platinum's outer electron configuration of 5d96s1. This is due to the relativistic stabilization of the 7s2 electron pair over the whole seventh period, so that none of the elements from 104 to 112 are expected to have electron configurations violating the Aufbau principle. The atomic radius of darmstadtium is expected to be around 132 pm.[2]
Experimental chemistry
Unambiguous determination of the chemical characteristics of darmstadtium has yet to have been established[34] due to the short half-lives of darmstadtium isotopes and a limited number of likely volatile compounds that could be studied on a very small scale. One of the few darmstadtium compounds that are likely to be sufficiently volatile is darmstadtium hexafluoride (DsF
6), as its lighter homologue platinum hexafluoride (PtF
6) is volatile above 60 °C and therefore the analogous compound of darmstadtium might also be sufficiently volatile;[15] a volatile octafluoride (DsF
8) might also be possible.[2] For chemical studies to be carried out on a transactinide, at least four atoms must be produced, the half-life of the isotope used must be at least 1 second, and the rate of production must be at least one atom per week.[15] Even though the half-life of 281Ds, the most stable confirmed darmstadtium isotope, is 11 seconds, long enough to perform chemical studies, another obstacle is the need to increase the rate of production of darmstadtium isotopes and allow experiments to carry on for weeks or months so that statistically significant results can be obtained. Separation and detection must be carried out continuously to separate out the darmstadtium isotopes and automated systems can then experiment on the gas-phase and solution chemistry of darmstadtium as the yields for heavier elements are predicted to be smaller than those for lighter elements; some of the separation techniques used for bohrium and hassium could be reused. However, the experimental chemistry of darmstadtium has not received as much attention as that of the heavier elements from copernicium to livermorium.[2][34][35]
The more neutron-rich darmstadtium isotopes are the most stable[17] and are thus more promising for chemical studies;[2][15] however, they can only be produced indirectly from the alpha decay of heavier elements,[6][24][25] and indirect synthesis methods are not favourable for chemical studies.[2] The more neutron-rich isotopes 276Ds and 277Ds might be produced directly in the reaction between thorium-232 and calcium-48, but the yield is expected to be low.[2][36][37] Furthermore, this reaction has already been tested without success,[36] and more recent experiments that have successfully synthesized 277Ds using indirect methods show that it has a short half-life of 5.7 ms, not long enough to perform chemical studies.[24]
See also
References
- ↑ "Darmstadtium". Periodic Table of Videos. The University of Nottingham. Retrieved 19 October 2012.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 Hoffman, Darleane C.; Lee, Diana M.; Pershina, Valeria (2006). "Transactinides and the future elements". In Morss; Edelstein, Norman M.; Fuger, Jean. The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. ISBN 1-4020-3555-1.
- 1 2 3 Östlin, A.; Vitos, L. (2011). "First-principles calculation of the structural stability of 6d transition metals". Physical Review B. 84 (11). Bibcode:2011PhRvB..84k3104O. doi:10.1103/PhysRevB.84.113104.
- 1 2 Fricke, Burkhard (1975). "Superheavy elements: a prediction of their chemical and physical properties". Recent Impact of Physics on Inorganic Chemistry. 21: 89–144. doi:10.1007/BFb0116498. Retrieved 4 October 2013.
- ↑ Chemical Data. Darmstadtium - Ds, Royal Chemical Society
- 1 2 3 Oganessian, Y. T.; Utyonkov, V.; Lobanov, Y.; Abdullin, F.; Polyakov, A.; Shirokovsky, I.; Tsyganov, Y.; Gulbekian, G.; Bogomolov, S.; Gikal, B.; et al. (2004). "Measurements of cross sections for the fusion-evaporation reactions 244Pu(48Ca,xn)292−x114 and 245Cm(48Ca,xn)293−x116". Physical Review C. 69 (5): 054607. Bibcode:2004PhRvC..69e4607O. doi:10.1103/PhysRevC.69.054607.
- 1 2 Hofmann, S.; Ninov, V.; Heßberger, F. P.; Armbruster, P.; Folger, H.; Münzenberg, G.; Schött, H. J.; Popeko, A. G.; Yeremin, A. V.; Andreyev, A. N.; Saro, S.; Janik, R.; Leino, M. (1995). "Production and decay of 269110". Zeitschrift für Physik A. 350 (4): 277. Bibcode:1995ZPhyA.350..277H. doi:10.1007/BF01291181.
- 1 2 3 Hofmann, S (1998). "New elements – approaching". Reports on Progress in Physics. 61 (6): 639. Bibcode:1998RPPh...61..639H. doi:10.1088/0034-4885/61/6/002.
- ↑ Barber, R. C.; Greenwood, N.N.; Hrynkiewicz, A.Z.; Jeannin, Y.P.; Lefort, M.; Sakai, M.; Ulehla, I.; Wapstra, A.P.; Wilkinson, D.H. (1993). "Discovery of the transfermium elements. Part II: Introduction to discovery profiles. Part III: Discovery profiles of the transfermium elements". Pure and Applied Chemistry. 65 (8): 1757. doi:10.1351/pac199365081757. (Note: for Part I see Pure Appl. Chem., Vol. 63, No. 6, pp. 879–886, 1991)
- ↑ Karol, P. J.; Nakahara, H.; Petley, B. W.; Vogt, E. (2001). "On the discovery of the elements 110–112 (IUPAC Technical Report)". Pure and Applied Chemistry. 73 (6): 959. doi:10.1351/pac200173060959.
- ↑ Chatt, J. (1979). "Recommendations for the naming of elements of atomic numbers greater than 100". Pure and Applied Chemistry. 51 (2): 381–384. doi:10.1351/pac197951020381.
- ↑ http://web.archive.org/web/20050117160457/http://element114.narod.ru/110-history.html
- ↑ https://books.google.ru/books?id=yP63CgAAQBAJ&pg=PA397&lpg=PA397&dq=element+110+hahnium&source=bl&ots=-VbpvQ0fky&sig=nQZpPlXPEnWI22Dz3kgGflMwriA&hl=ru&sa=X&ved=0ahUKEwjPgtH-kavQAhXGiiwKHWhHDBoQ6AEIMzAD#v=onepage&q&f=false
- 1 2 Corish, J.; Rosenblatt, G. M. (2003). "Name and symbol of the element with atomic number 110" (PDF). Pure Appl. Chem. 75 (10): 1613–1615. doi:10.1351/pac200375101613. Retrieved 17 October 2012.
- 1 2 3 4 5 Griffith, W. P. (2008). "The Periodic Table and the Platinum Group Metals". Platinum Metals Review. 52 (2): 114. doi:10.1595/147106708X297486.
- ↑ "Chemistry in its element – darmstadtium". Chemistry in its element. Royal Society of Chemistry. Retrieved 17 October 2012.
- 1 2 3 4 5 6 7 8 Sonzogni, Alejandro. "Interactive Chart of Nuclides". National Nuclear Data Center: Brookhaven National Laboratory. Retrieved 2008-06-06.
- 1 2 3 Gray, Theodore (2002–2010). "The Photographic Periodic Table of the Elements". periodictable.com. Retrieved 16 November 2012.
- 1 2 http://xxx.lanl.gov/pdf/1502.03030.pdf
- ↑ Ghiorso, A.; Lee, D.; Somerville, L.; Loveland, W.; Nitschke, J.; Ghiorso, W.; Seaborg, G.; Wilmarth, P.; Leres, R.; Wydler, A.; Nurmia, M.; Gregorich, K.; Czerwinski, K.; Gaylord, R.; Hamilton, T.; Hannink, N. J.; Hoffman, D. C.; Jarzynski, C.; Kacher, C.; Kadkhodayan, B.; Kreek, S.; Lane, M.; Lyon, A.; McMahan, M. A.; Neu, M.; Sikkeland, T.; Swiatecki, W. J.; Türler, A.; Walton, J. T.; Yashita, S. (1995). "Evidence for the possible synthesis of element 110 produced by the 59Co+209Bi reaction". Physical Review C. 51 (5): R2293. Bibcode:1995PhRvC..51.2293G. doi:10.1103/PhysRevC.51.R2293.
- 1 2 Hofmann; Heßberger, F.P.; Ackermann, D.; Antalic, S.; Cagarda, P.; Ćwiok, S.; Kindler, B.; Kojouharova, J.; Lommel, B.; Mann, R.; Münzenberg, G.; Popeko, A.G.; Saro, S.; Schött, H.J.; Yeremin, A.V. (2001). "The new isotope 270110 and its decay products 266Hs and 262Sg" (PDF). Eur. Phys. J. A. 10: 5–10. Bibcode:2001EPJA...10....5H. doi:10.1007/s100500170137.
- ↑ Lazarev, Yu. A.; Lobanov, Yu.; Oganessian, Yu.; Utyonkov, V.; Abdullin, F.; Polyakov, A.; Rigol, J.; Shirokovsky, I.; Tsyganov, Yu.; Iliev, S.; Subbotin, V. G.; Sukhov, A. M.; Buklanov, G. V.; Gikal, B. N.; Kutner, V. B.; Mezentsev, A. N.; Subotic, K.; Wild, J. F.; Lougheed, R. W.; Moody, K. J. (1996). "α decay of 273110: Shell closure at N=162". Physical Review C. 54 (2): 620. Bibcode:1996PhRvC..54..620L. doi:10.1103/PhysRevC.54.620.
- 1 2 Dullman, C.E. Superheavy Element Research Superheavy Element - News from GSI and Mainz. University Mainz
- 1 2 3 Public Affairs Department (26 October 2010). "Six New Isotopes of the Superheavy Elements Discovered: Moving Closer to Understanding the Island of Stability". Berkeley Lab. Retrieved 2011-04-25.
- 1 2 Yeremin, A. V.; et al. (1999). "Synthesis of nuclei of the superheavy element 114 in reactions induced by 48Ca". Nature. 400 (6741): 242. Bibcode:1999Natur.400..242O. doi:10.1038/22281.
- ↑ Nie, G. K. (2005). "Charge radii of β-stable nuclei". Modern Physics Letters A. 21 (24): 1889. arXiv:nucl-th/0512023
 . Bibcode:2006MPLA...21.1889N. doi:10.1142/S0217732306020226.
. Bibcode:2006MPLA...21.1889N. doi:10.1142/S0217732306020226. - ↑ P. Roy Chowdhury; C. Samanta & D. N. Basu (2006). "α decay half-lives of new superheavy elements". Phys. Rev. C. 73: 014612. arXiv:nucl-th/0507054
 . Bibcode:2006PhRvC..73a4612C. doi:10.1103/PhysRevC.73.014612.
. Bibcode:2006PhRvC..73a4612C. doi:10.1103/PhysRevC.73.014612. - ↑ C. Samanta; P. Roy Chowdhury & D.N. Basu (2007). "Predictions of alpha decay half lives of heavy and superheavy elements". Nucl. Phys. A. 789: 142–154. arXiv:nucl-th/0703086
 . Bibcode:2007NuPhA.789..142S. doi:10.1016/j.nuclphysa.2007.04.001.
. Bibcode:2007NuPhA.789..142S. doi:10.1016/j.nuclphysa.2007.04.001. - ↑ P. Roy Chowdhury; C. Samanta & D. N. Basu (2008). "Search for long lived heaviest nuclei beyond the valley of stability". Phys. Rev. C. 77 (4): 044603. arXiv:0802.3837
 . Bibcode:2008PhRvC..77d4603C. doi:10.1103/PhysRevC.77.044603.
. Bibcode:2008PhRvC..77d4603C. doi:10.1103/PhysRevC.77.044603. - ↑ P. Roy Chowdhury; C. Samanta & D. N. Basu (2008). "Nuclear half-lives for α -radioactivity of elements with 100 ≤ Z ≤ 130". Atomic Data and Nuclear Data Tables. 94 (6): 781. arXiv:0802.4161
 . Bibcode:2008ADNDT..94..781C. doi:10.1016/j.adt.2008.01.003.
. Bibcode:2008ADNDT..94..781C. doi:10.1016/j.adt.2008.01.003. - ↑ Rosen, A.; Fricke, B.; Morovic, T.; Ellis, D. E. (1979). J. Phys. C4, Suppl. 4. 40: C4/218–219. Missing or empty
|title=(help) - ↑ Waber, J. T.; Averill, F. W. (1974). "Molecular orbitals of PtF6 and E110 F6 calculated by the self-consistent multiple scattering Xα method". J. Chem. Phys. 60 (11): 4460–70. Bibcode:1974JChPh..60.4466W. doi:10.1063/1.1680924.
- 1 2 Thayer, John S. (2010). "Relativistic Effects and the Chemistry of the Heavier Main Group Elements": 82. doi:10.1007/978-1-4020-9975-5_2.
- 1 2 Düllmann, Christoph E. (2012). "Superheavy elements at GSI: a broad research program with element 114 in the focus of physics and chemistry". Radiochimica Acta. 100 (2): 67–74. doi:10.1524/ract.2011.1842.
- ↑ Eichler, Robert (2013). "First foot prints of chemistry on the shore of the Island of Superheavy Elements" (PDF). Journal of Physics: Conference Series. IOP Science. 420 (1). arXiv:1212.4292
 . Bibcode:2013JPhCS.420a2003E. doi:10.1088/1742-6596/420/1/012003. Retrieved 11 September 2014.
. Bibcode:2013JPhCS.420a2003E. doi:10.1088/1742-6596/420/1/012003. Retrieved 11 September 2014. - 1 2 Flerov lab annual report 2004
- ↑ Feng, Z; Jin, G; Li, J; Scheid, W (2009). "Production of heavy and superheavy nuclei in massive fusion reactions". Nuclear Physics A. 816: 33. arXiv:0803.1117
 . Bibcode:2009NuPhA.816...33F. doi:10.1016/j.nuclphysa.2008.11.003.
. Bibcode:2009NuPhA.816...33F. doi:10.1016/j.nuclphysa.2008.11.003.
External links
- Darmstadtium at The Periodic Table of Videos (University of Nottingham)
| Periodic table (Large cells) | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||||||||||||||||
| 1 | H | He | |||||||||||||||||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | |||||||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||||||||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |||||||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |||||||||||||||
| 6 | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | |
| 7 | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
|
| |||||||||||||||||||||||||||||||||