Tennessine
| General properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, symbol | tennessine, Ts | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pronunciation |
/ˈtɛnᵻsaɪn/ or /ˈtɛnᵻsiːn/ TEN-ə-seen or TEN-ə-syn | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | semimetallic (predicted)[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tennessine in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 117 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, block | group 17, p-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | unknown, but probably a post-transition metal[2][3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight (Ar) | [294] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f14 6d10 7s2 7p5 (predicted)[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
per shell | 2, 8, 18, 32, 32, 18, 7 (predicted) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase | solid (predicted)[4][5] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 623–823 K (350–550 °C, 662–1022 °F) (predicted)[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 883 K (610 °C, 1130 °F) (predicted)[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density near r.t. | 7.1–7.3 g/cm3 (extrapolated)[5] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −1, +1, +3, +5 (predicted)[1][4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
1st: 742.9 kJ/mol (predicted)[4] 2nd: 1785.0–1920.1 kJ/mol (extrapolated)[5] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 138 pm (predicted)[5] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 156–157 pm (extrapolated)[5] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 54101-14-3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Naming | after Tennessee region | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Joint Institute for Nuclear Research and Lawrence Livermore National Laboratory (2010) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Most stable isotopes of tennessine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Tennessine is a superheavy artificial chemical element with an atomic number of 117 and a symbol of Ts. Also known as eka-astatine or element 117, it is the second-heaviest known element and penultimate element of the 7th period of the periodic table. As of 2016, fifteen tennessine atoms have been observed: six when it was first synthesized in 2010, seven in 2012, and two in 2014.
The discovery of tennessine was announced in Dubna, Russia, by a Russian–American collaboration in 2010, which makes it the most recently discovered element as of 2016. One of its daughter isotopes was created directly in 2011, partially confirming the results of the experiment. The experiment itself was repeated successfully by the same collaboration in 2012 and by a joint German–American team in 2014. In 2015, the Joint Working Party of the International Union of Pure and Applied Chemistry and the International Union of Pure and Applied Physics, which evaluates claims of discovery of new elements, has recognized the element and assigned the priority to the Russian–American team. In June 2016, the IUPAC published a declaration stating that the discoverers suggested the name tennessine after Tennessee, United States;[lower-alpha 1] On 28 November 2016, the IUPAC officially adopted the name "tennessine".[8][9]
Tennessine may be located in the "island of stability", a concept that explains why some superheavy elements are more stable compared to an overall trend of decreasing stability for elements beyond bismuth on the periodic table. The synthesized tennessine atoms have lasted tens and hundreds of milliseconds. In the periodic table, tennessine is expected to be a member of group 17, all other members of which are halogens.[lower-alpha 2] Some of its properties may significantly differ from those of the halogens due to relativistic effects. As a result, tennessine is expected to be a volatile metal that neither forms anions nor achieves high oxidation states. A few key properties, such as its melting and boiling points and its first ionization energy, are nevertheless expected to follow the periodic trends of the halogens.
History
Pre-discovery
In December 2004, the Joint Institute for Nuclear Research (JINR) team in Dubna, Moscow Oblast, Russia, proposed a joint experiment with the Oak Ridge National Laboratory (ORNL) in Oak Ridge, Tennessee, United States, to synthesize element 117—so-called for the 117 protons in its nucleus. Their proposal involved fusing a berkelium (element 97) target and a calcium (element 20) beam, conducted via bombardment of the berkelium target with calcium nuclei.[11] The ORNL—then the world's only producer of berkelium—could not then provide the element, as they had temporarily ceased production,[11] and re-initiating it would be too costly.[12] Plans to synthesize element 117 were suspended in favor of the synthesis of element 118, which was produced earlier by bombarding a californium target with calcium.[13] The required berkelium-249 is a by-product in californium-252 production, and obtaining the required amount of berkelium was an even more difficult task than obtaining that of californium, as well as costly: it would cost around 3.5 million dollars, and the parties agreed to wait for a commercial order of californium production, from which berkelium could be extracted.[12][14]
The JINR team sought to use berkelium because calcium-48, the isotope of calcium used in the beam, has 20 protons and 28 neutrons, making a neutron–proton ratio of 1.4; and it is the lightest stable or near-stable nucleus with such a large neutron excess. The second-lightest such nucleus, palladium-110 (46 protons, 64 neutrons, neutron–proton ratio of 1.391), is much heavier. Thanks to the neutron excess, the resulting nuclei were expected to be heavier and closer to the sought-after island of stability.[lower-alpha 3] Of the aimed for 117 protons, calcium has 20, and thus they needed to use berkelium, which has 97 protons in its nucleus.[15]
In February 2005, the leader of the JINR team—Yuri Oganessian—held a colloquium at ORNL and attended a symposium at Vanderbilt University (Nashville, Tennessee, U.S.) celebrating the career of a long-standing collaborator of Oganessian, Joseph Hamilton, where Oganessian, Hamilton, and James Roberto (then the deputy director for science and technology at Oak Ridge) established a collaboration between JINR, ORNL, and Vanderbilt;[14] the team at the Lawrence Livermore National Laboratory (LLNL) in Livermore, California, U.S., was soon invited to join.[17] Hamilton checked if the ORNL high-flux reactor produced californium for a commercial order: the required berkelium could be obtained as a by-product. He learned that it did not and there was no expectation for such an order in immediate future. Hamilton kept monitoring the situation, making the checks once in a while. (Later, Oganessian referred to Hamilton as "the father of 117" for doing this work.)[18]
Discovery
In the spring of 2008, the ORNL started production of californium for a commercial order by an oil company;[14] Hamilton learned that in the summer, making a deal on subsequent extraction of berkelium.[19] In September 2008, Oganessian, Hamilton, and Roberto assembled to coordinate planning on the experiment.[17] In November 2008, the U.S. Department of Energy, the state department oversight over the reactor in Oak Ridge, allowed the scientific use of the extracted berkelium.[17] The production lasted 250 days and ended in late December 2008,[20] resulting in 22 milligrams of berkelium, enough to perform the experiment.[21] In January 2009, the berkelium was removed from the reactor;[17] it was subsequently cooled for 90 days and then chemically purified for another 90 days.[14] Its half-life is only 330 days: after that time, half the berkelium produced would have decayed. Because of this, the berkelium target had to be quickly transported to Russia; for the experiment to be viable, it had to begin within six months of its departure from the United States.[14] The target was packed into five lead containers to be sent on a commercial flight from New York to Moscow.[14]
Russian customs officials twice refused to let the target enter the country because of missing or incomplete paperwork. Over the span of a few days, the target traveled over the Atlantic Ocean five times.[14] On its arrival in Russia in early June 2009, the berkelium was transferred to Research Institute of Atomic Reactors (RIAR) in Dimitrovgrad, Ulyanovsk Oblast, where it was deposited as a 300-nanometer-thin layer on a titanium film.[20] In July 2009, it was then transported to Dubna,[20] where it was installed in the particle accelerator at JINR.[21] The calcium-48 beam was generated by chemically extracting the small quantities of calcium-48 present in naturally occurring calcium,[22] enriching it 500 times in the closed town of Lesnoy, Sverdlovsk Oblast, Russia.[17]
The experiment began later in July 2009.[17] In January 2010, scientists at the Flerov Laboratory of Nuclear Reactions announced internally that they had detected the decay of a new element with atomic number 117 via two decay chains: one of an odd-odd isotope undergoing 6 alpha decays before spontaneous fission, and one of an odd-even isotope undergoing 3 alpha decays before fission.[23] The obtained data from the experiment was sent to the LLNL for further analysis.[24] On April 9, 2010, an official report was released in the journal Physical Review Letters identifying the isotopes as 294117 and 293117, which were shown to have half-lives on the order of tens or hundreds of milliseconds. The work was signed by all parties involved in the experiment to some extent: JINR, ORNL, LLNL, RIAR, Vanderbilt, the University of Tennessee, and the University of Nevada (Las Vegas, Nevada, U.S.), which provided data analysis support.[25] The isotopes were formed as follows:[26][lower-alpha 4]
- 249
97Bk
+ 48
20Ca
→ 297117 → 294117 + 3 1
0
n
(1 event)
- 249
97Bk
+ 48
20Ca
→ 297117* → 293117 + 4 1
0
n
(5 events)
Confirmation
All daughter isotopes (decay products) of element 117 were previously unknown;[26] therefore, their properties could not be used to confirm the claim of discovery. In 2011, when one of the decay products was synthesized directly, its properties matched those measured in the claimed indirect synthesis from the decay of element 117.[27] The discoverers did not submit a claim for their findings in 2007–2011 when the Joint Working Party was reviewing claims of discoveries of new elements.[28]
The Dubna team repeated the experiment in 2012, creating seven atoms of element 117 and confirming their earlier synthesis of element 118 (produced after some time when a significant quantity of the berkelium-249 target had beta decayed to californium-249). The results of the experiment matched the previous outcome;[6] the scientists have since filed an application to register the element.[29] In 2014, a joint German–American collaboration of scientists from the ORNL and the GSI Helmholtz Center for Heavy Ion Research in Darmstadt, Germany, claimed to have confirmed discovery of the element.[7][30] The team repeated the Dubna experiment using the Darmstadt accelerator, creating two atoms of element 117.[7]
In December 2015, the JWP officially recognized the discovery,[31] and thus the listed discoverers—JINR, LLNL, and ORNL—were given the right to suggest an official name for the element.[29]
Naming
Using Mendeleev's nomenclature for unnamed and undiscovered elements, element 117 should be known as eka-astatine. Using the 1979 IUPAC recommendations, the element was temporarily called ununseptium (symbol Uus) until its discovery was confirmed and a permanent name chosen; the temporary name was formed from Latin roots "one", "one", and "seven", a reference to the element's atomic number of 117.[32] Many scientists in the field called it "element 117", with the symbol (117) or 117.[4] According to guidelines of the International Union of Pure and Applied Chemistry (IUPAC) valid at the moment of the discovery approval, the permanent names of new elements should have ended in "-ium"; this included element 117, even if the element was a halogen, which traditionally have names ending in "-ine";[33] however, the new recommendations published in 2016 recommended using the "-ine" ending for all new group 17 elements.[34]
After the original synthesis in 2010, Dawn Shaughnessy of LLNL and Oganessian declared naming was a sensitive question, and it was avoided as far as possible.[35] However, Hamilton declared that year, "I was crucial in getting the group together and in getting the 249Bk target essential for the discovery. As a result of that, I’m going to get to name the element. I can’t tell you the name, but it will bring distinction to the region" (Hamilton's institute of Vanderbilt is located in Nashville, Tennessee, U.S.).[25]
| Wikinews has related news: IUPAC proposes four new chemical element names |
In June 2016, IUPAC published a declaration stating the discoverers submitted their suggestions for naming the new elements to the IUPAC; the suggestion for the element 117 was tennessine, with a symbol of Ts, after "the region of Tennessee".[lower-alpha 1] The announcement by the IUPAC also confirmed Vanderbilt as another discoverer institute. The names were recommended for acceptance by the IUPAC Inorganic Chemistry Division; formal acceptance was set to occur after a five-months term following publising of the declaration expires.[36] In November 2016, the name was formally accepted. Concerns that the proposed symbol Ts may clash with a notation for the tosyl group used in organic chemistry were rejected, following existing symbols bearing such dual meanings: Ac (actinium and acetyl) and Pr (praseodymium and propyl).[37]
Predicted properties
Nuclear stability and isotopes
The stability of nuclei quickly decreases with the increase in atomic number after curium, element 96, whose half-life is four orders of magnitude longer than that of any subsequent element. All isotopes with an atomic number above 101 undergo radioactive decay with half-lives of less than 30 hours. No elements with atomic numbers above 82 (after lead) have stable isotopes.[38] Nevertheless, because of reasons not yet well understood, there is a slight increase of nuclear stability around atomic numbers 110–114, which leads to the appearance of what is known in nuclear physics as the "island of stability". This concept, proposed by University of California professor Glenn Seaborg, explains why superheavy elements last longer than predicted.[39] Tennessine is the second-heaviest element created so far, and has a half-life of less than one second; this is longer than the predicted value used in the discovery report.[26] The Dubna team believes that the synthesis of the element is direct experimental proof of the existence of the island of stability.[40]
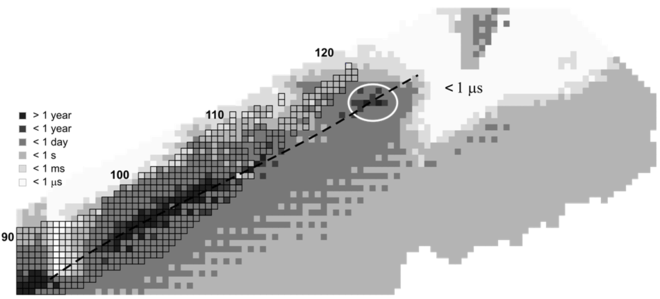
It has been calculated that the isotope 295Ts would have a half-life of 18 ± 7 milliseconds and that it may be possible to produce this isotope via the same berkelium–calcium reaction used in the discoveries of the known isotopes, 293Ts and 294Ts. The chance of this reaction producing 295Ts is estimated to be, at most, one-seventh the chance of producing 294Ts.[41][42][43] Calculations using a quantum tunneling model predict the existence of several isotopes of tennessine up to 303Ts. The most stable of these is expected to be 296Ts with an alpha-decay half-life of 40 milliseconds.[44] A liquid drop model study on the element's isotopes shows similar results; it suggests a general trend of increasing stability for isotopes heavier than 301Ts, with partial half-lives exceeding the age of the universe for the heaviest isotopes like 335Ts when beta decay is not considered.[45]
Atomic and physical
Tennessine is expected to be a member of group 17 in the periodic table, below the five halogens; fluorine, chlorine, bromine, iodine, and astatine, each of which has seven valence electrons with a configuration of ns2np5.[46][lower-alpha 5] For tennessine, being in the seventh period (row) of the periodic table, continuing the trend would predict a valence electron configuration of 7s27p5,[4] and it would therefore be expected to behave similarly to the halogens in many respects that relate to this electronic state. However, going down group 17, the metallicity of the elements increases; for example, iodine already exhibits a metallic luster in the solid state, and astatine is often classified as a metalloid due to its properties being quite far from those of the four previous halogens. As such, an extrapolation based on periodic trends would predict tennessine to be a rather volatile post-transition metal.[3]
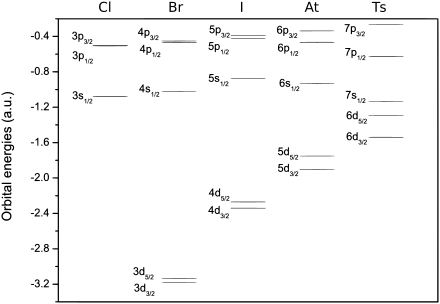
Calculations have confirmed the accuracy of this simple extrapolation, although experimental verification of this is currently impossible as the half-lives of the known tennessine isotopes are too short.[3] Significant differences between tennessine and the previous halogens are likely to arise, largely due to spin–orbit interaction — the mutual interaction between the motion and spin of electrons. The spin–orbit interaction is especially strong for the superheavy elements because their electrons move faster — at velocities comparable to the speed of light — than those in lighter atoms.[47] In tennessine atoms, this lowers the 7s and the 7p electron energy levels, stabilizing the corresponding electrons, although two of the 7p electron energy levels are more stabilized than the other four.[48] The stabilization of the 7s electrons is called the inert pair effect; the effect that separates the 7p subshell into the more-stabilized and the less-stabilized parts is called subshell splitting. Computational chemists understand the split as a change of the second (azimuthal) quantum number l from 1 to 1/2 and 3/2 for the more-stabilized and less-stabilized parts of the 7p subshell, respectively.[49][lower-alpha 6] For many theoretical purposes, the valence electron configuration may be represented to reflect the 7p subshell split as 7s2
7p2
1/27p3
3/2.[4]
Differences for other electron levels also exist. For example, the 6d electron levels (also split in two, with four being 6d3/2 and six being 6d5/2) are both raised, so they are close in energy to the 7s ones,[48] although no 6d electron chemistry has been predicted for tennessine. The difference between the 7p1/2 and 7p3/2 levels is abnormally high; 9.8 eV.[48] Astatine's 6p subshell split is only 3.8 eV,[48] and its 6p1/2 chemistry has already been called "limited".[50] These effects cause tennessine's chemistry to differ from those of its upper neighbors (see below).
Tennessine's first ionization energy—the energy required to remove an electron from a neutral atom—is predicted to be 7.7 eV, lower than those of the halogens, again following the trend.[4] Like its neighbors in the periodic table, tennessine is expected to have the lowest electron affinity—energy released when an electron is added to the atom—in its group; 2.6 or 1.8 eV.[4] The electron of the hypothetical hydrogen-like tennessine atom—oxidized so it has only one electron, Ts116+—is predicted to move so quickly that its mass is 1.9 times that of a non-moving electron, a feature attributable to relativistic effects. For comparison, the figure for hydrogen-like astatine is 1.27 and the figure for hydrogen-like iodine is 1.08.[51] Simple extrapolations of relativity laws indicate a contraction of atomic radius.[51] Advanced calculations show that the radius of an tennessine atom that has formed one covalent bond would be 165 pm, while that of astatine would be 147 pm.[52] With the seven outermost electrons removed, tennessine is finally smaller; 57 pm[4] for tennessine and 61 pm[53] for astatine.
The melting and boiling points of tennessine are not known; earlier papers predicted about 350–500 °C and 550 °C, respectively,[4] or 350–550 °C and 610 °C, respectively.[54] These values exceed those of astatine and the lighter halogens, following periodic trends. A later paper predicts the boiling point of tennessine to be 345 °C[55] (that of astatine is estimated as 309 °C,[56] 337 °C,[57] or 370 °C,[58] although experimental values of 230 °C[59] and 411 °C[53] have been reported). The density of tennessine is expected to be between 7.1 and 7.3 g·cm−3, continuing the trend of increasing density among the halogens; that of astatine is estimated to be between 6.2 and 6.5 g·cm−3.[5]
Chemical
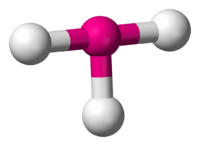
3 has a T-shape configuration.
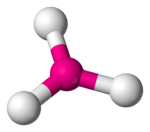
3 is predicted to have a trigonal one.
Unlike the previous group 17 elements, tennessine may not exhibit chemical behavior common to the halogens.[10] For example, the extant members of the group routinely accept an electron to achieve the more stable electronic configuration of a noble gas, one having eight electrons (octet) in its valence shell.[60] This ability weakens as atomic weight increases going down the group; tennessine would be the least willing to accept an electron. Of the oxidation states it is predicted to form, −1 is expected to be the least common.[4] The standard reduction potential of the Ts/Ts− couple is predicted to be −0.25 V; this value is negative and thus tennessine should not be reduced to the −1 oxidation state under standard conditions, unlike all the previous halogens.[1]
There is another opportunity for tennessine to complete its octet—by forming a covalent bond. Like the halogens, when two tennessine atoms meet they are expected to form a Ts–Ts bond to give a diatomic molecule. Such molecules are commonly bound via single sigma bonds between the atoms; these are different from pi bonds, which are divided into two parts, each shifted in a direction perpendicular to the line between the atoms, and opposite one another rather than being located directly between the atoms they bind. Sigma bonding has been calculated to show a great antibonding character in the At2 molecule and is not as favorable energetically. Tennessine is predicted to continue the trend; a strong pi character should be seen in the bonding of Ts2.[4][61] The molecule tennessine chloride (TsCl)[lower-alpha 7] is predicted to go further, being bonded with a single pi bond.[61]
Aside from the unstable −1 state, three more oxidation states are predicted; +5, +3, and +1. The +1 state should be especially stable because of the destabilization of the three outermost 7p3/2 electrons, forming a stable, half-filled subshell configuration;[4] astatine shows similar effects.[62] The +3 state should be important, again due to the destabilized 7p3/2 electrons.[54] The +5 state is predicted to be uncommon because the 7p1/2 electrons are oppositely stabilized.[4] The +7 state has not been shown—even computationally—to be achievable. Because the 7s electrons are greatly stabilized, it has been hypothesized that tennessine effectively has only five valence electrons.[63]
The simplest possible tennessine compound would be the monohydride, TsH. The bonding is expected to be provided by a 7p3/2 electron of tennessine and the 1s electron of hydrogen. The non-bonding nature of the 7p1/2 spinor is because tennessine is expected not to form purely sigma or pi bonds.[64] Therefore, the destabilized (thus expanded) 7p3/2 spinor is responsible for bonding.[65] This effect lengthens the TsH molecule by 17 picometers compared with the overall length of 195 pm.[64] Since the tennessine p electron bonds are two-thirds sigma, the bond is only two-thirds as strong as it would be if tennessine featured no spin–orbit interactions.[64] The molecule thus follows the trend for halogen hydrides, showing an increase in bond length and a decrease in dissociation energy compared to AtH.[4] The molecules TlTs and NhTs may be viewed analogously, taking into account an opposite effect shown by the fact that the element's p1/2 electrons are stabilized. These two characteristics result in a relatively small dipole moment (product of difference between electric charges of atoms and displacement of the atoms) for TlTs; only 1.67 D,[lower-alpha 8] the positive value implying that the negative charge is on the tennessine atom. For NhTs, the strength of the effects are predicted to cause a transfer of the electron from the tennessine atom to the nihonium atom, with the dipole moment value being −1.80 D.[67] The spin–orbit interaction increases the dissociation energy of the TsF molecule because it lowers the electronegativity of tennessine, causing the bond with the extremely electronegative fluorine atom to have a more ionic character.[64] Tennessine monofluoride should feature the strongest bonding of all group 17 element monofluorides.[64]
VSEPR theory predicts a bent-T-shaped molecular geometry for the group 17 trifluorides. All known halogen trifluorides have this molecular geometry and have a structure of AX3E2—a central atom, denoted A, surrounded by three ligands, X, and two unshared electron pairs, E. If relativistic effects are ignored, TsF3 should follow its lighter congeners in having a bent-T-shaped molecular geometry. More sophisticated predictions show that this molecular geometry would not be energetically favored for TsF3, predicting instead a trigonal planar molecular geometry (AX3E0). This shows that VSEPR theory may not be consistent for the superheavy elements.[63] The TsF3 molecule is predicted to be significantly stabilized by spin–orbit interactions; a possible rationale may be the large difference in electronegativity between tennessine and fluorine, giving the bond a partially ionic character.[63]
Notes
- 1 2 The declaration by the IUPAC mentioned "the contribution of the Tennessee region (emphasis added), including Oak Ridge National Laboratory, Vanderbilt University, and the University of Tennessee at Knoxville, to superheavy element research, including the production and chemical separation of unique actinide target materials for superheavy element synthesis at ORNL’s High Flux Isotope Reactor (HFIR) and Radiochemical Engineering Development Center (REDC)".
- ↑ The term "group 17" refers to a column in the periodic table starting with fluorine and is distinct from "halogen", which relates to a common set of chemical and physical properties shared by fluorine, chlorine, bromine, iodine, and astatine, all of which precede tennessine in group 17. Unlike the other group 17 members, tennessine may not be a halogen.[10]
- ↑ Although stable isotopes of the lightest elements usually have a neutron–proton ratio close or equal to one (for example, the only stable isotope of aluminium has 13 protons and 14 neutrons,[15] making a neutron–proton ratio of 1.077), stable isotopes of heavier elements have higher neutron–proton ratios, increasing with the number of protons. For example, iodine's only stable isotope has 53 protons and 74 neutrons, giving neutron–proton ratio of 1.396, gold's only stable isotope has 79 protons and 118 neutrons, yielding a neutron–proton ratio of 1.494, and plutonium's most stable isotope has 94 protons and 150 neutrons, and a neutron–proton ratio of 1.596.[15] This trend[16] is expected to make it difficult to synthesize the most stable isotopes of super-heavy elements as the neutron–proton ratios of the elements they are synthesized from will be too low.
- ↑ A nuclide is commonly denoted by the chemical element's symbol immediately preceded by the mass number as a superscript and the atomic number as a subscript. Neutrons are represented as nuclides with atomic mass 1, atomic number 0, and symbol n. Outside the context of nuclear equations, the atomic number is sometimes omitted. An asterisk denotes an extremely short-lived (or even non-existent) intermediate stage of the reaction.
- ↑ The letter n stands for the number of the period (horizontal row in the periodic table) the element belongs to. The letters "s" and "p" denote the s and p atomic orbitals, and the subsequent superscript numbers denote the numbers of electrons in each. Hence the notation ns2np5 means that the valence shells of lighter group 17 elements are composed of two s electrons and five p electrons, all located in the outermost electron energy level.
- ↑ The quantum number corresponds to the letter in the electron orbital name: 0 to s, 1 to p, 2 to d, etc. See azimuthal quantum number for more information.
- ↑ This is not to be confused with the most common usage of the formula "TsCl", the common organic reagent tosyl chloride.
- ↑ For comparison, the values for the ClF, HCl, SO, HF, and HI molecules are 0.89 D, 1.11 D, 1.55 D, 1.83 D, and 1.95 D. Values for molecules which do not form at standard conditions namely GeSe, SnS, TlF, BaO, and NaCl, are 1.65 D, ~3.2 D, 4.23 D, 7.95 D, and 9.00 D.[66]
References
- 1 2 3 Fricke, B. (1975). "Superheavy elements: a prediction of their chemical and physical properties". Recent Impact of Physics on Inorganic Chemistry. 21: 89–144. doi:10.1007/BFb0116498. Retrieved 4 October 2013.
- ↑ Royal Society of Chemistry (2016). "Ununseptium". rsc.org. Royal Society of Chemistry. Retrieved 9 November 2016.
A highly radioactive metal, of which only a few atoms have ever been made.
- 1 2 3 GSI (14 December 2015). "Research Program – Highlights". superheavies.de. GSI. Retrieved 9 November 2016.
If this trend were followed, element 117 would likely be a rather volatile metal. Fully relativistic calculations agree with this expectation, however, they are in need of experimental confirmation.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Hoffman, D. C.; Lee, D. M.; Pershina, V. (2006). "Transactinides and the future elements". In Morss; Edelstein, N. M.; Fuger, J. The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Springer Science+Business Media. pp. 1652–1752. ISBN 1-4020-3555-1.
- 1 2 3 4 5 6 Bonchev, D.; Kamenska, V. (1981). "Predicting the Properties of the 113–120 Transactinide Elements". Journal of Physical Chemistry. 85 (9): 1177–1186. doi:10.1021/j150609a021.
- 1 2 Oganessian, Yu. Ts.; et al. (2013). "Experimental studies of the 249Bk + 48Ca reaction including decay properties and excitation function for isotopes of element 117, and discovery of the new isotope 277Mt". Physical Review C. 87 (5): 054621. Bibcode:2013PhRvC..87e4621O. doi:10.1103/PhysRevC.87.054621.
- 1 2 3 Khuyagbaatar, J.; Yakushev, A.; Düllmann, Ch. E.; et al. (2014). "48Ca+249Bk Fusion Reaction Leading to Element Z=117: Long-Lived α-Decaying 270Db and Discovery of 266Lr". Physical Review Letters. 112 (17): 172501. doi:10.1103/PhysRevLett.112.172501.
- ↑ Staff (30 November 2016). "IUPAC Announces the Names of the Elements 113, 115, 117, and 118". IUPAC. Retrieved 1 December 2016.
- ↑ St. Fleur, Nicholas (1 December 2016). "Four New Names Officially Added to the Periodic Table of Elements". New York Times. Retrieved 1 December 2016.
- 1 2 "Superheavy Element 117 Confirmed - On the Way to the "Island of Stability"". GSI Helmholtz Centre for Heavy Ion Research. Retrieved 2015-07-26.
- 1 2 Gabage, B. (2010). "International team discovers element 117". Oak Ridge National Laboratory. Retrieved 2015-07-28.
- 1 2 "Vanderbilt physicist plays pivotal role in discovery of new super-heavy element". Vanderbilt University. 2010. Retrieved 2016-06-12.
- ↑ Oganessian, Yu. Ts.; Utyonkov, V. K.; Lobanov, Yu. V.; et al. (2002). "Results from the first 249Cf+48Ca experiment" (PDF). JINR Communication. Retrieved 2015-09-23.
- 1 2 3 4 5 6 7 Bardi, J. S. (2010). "An Atom at the End of the Material World". Inside Science. Retrieved 2015-01-03.
- 1 2 3 Audi, G.; Bersillon, O.; Blachot, J.; et al. (2003). "The NUBASE evaluation of nuclear and decay properties" (PDF). Nuclear Physics A. 729 (1): 3–128. Bibcode:2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001.
- ↑ Karpov, A. V.; Zagrebaev, V. I.; Palenzuela, Y. Martinez; Greiner, Walter (2013). "Superheavy Nuclei: Decay and Stability". Exciting Interdisciplinary Physics. FIAS Interdisciplinary Science Series. p. 69. doi:10.1007/978-3-319-00047-3_6. ISBN 978-3-319-00046-6.
- 1 2 3 4 5 6 James Roberto (2010). The Discovery of Element 117 (PDF) (Report). Oak Ridge National Laboratory. Retrieved 2016-06-12.
- ↑ "What it takes to make a new element". Chemistry World. Retrieved 2016-12-03.
- ↑ Witze, Alexandra (2010). "The backstory behind a new element". Science News. Retrieved 2016-06-12.
- 1 2 3 Joint Institute for Nuclear Research (2010). For the Press (Report). Retrieved 2015-07-28.
- 1 2 Stark, A. M. (2010). "International team discovers element 117". DOE/Lawrence Livermore National Laboratory. Retrieved 2012-11-29.
- ↑ "Ununseptium – The 117th element". Sputnik. 2009-10-28. Retrieved 2012-07-07.
- ↑ Greiner, W. (2010). "Recommendations: 31st meeting, PAC for nuclear physics" (PDF): 6.
- ↑ U.S. DOE Office of Science (2011). "Nations Work Together to Discover New Element". U.S. Department of Energy. Retrieved 2016-01-05.
- 1 2 "Arts and Science Magazine » » Heaviest in the World". www.vanderbilt.edu. Retrieved 2016-06-12.
- 1 2 3 4 Oganessian, Yu. Ts.; Abdullin, F. Sh.; Bailey, P. D.; et al. (2010). "Synthesis of a New Element with Atomic Number Z=117". Physical Review Letters. American Physical Society. 104 (142502). Bibcode:2010PhRvL.104n2502O. doi:10.1103/PhysRevLett.104.142502. PMID 20481935.
- ↑ Molchanov, E. (2011). В лабораториях ОИЯИ. Возвращение к дубнию [In JINR labs. Returning to dubnium] (in Russian). JINR. Retrieved 2011-11-09.
- ↑ Barber, R. C.; Karol, P. J.; Nakahara, H.; et al. (2011). "Discovery of the elements with atomic numbers greater than or equal to 113 (IUPAC Technical Report)". Pure and Applied Chemistry. 83 (7): 1485–1498. doi:10.1351/PAC-REP-10-05-01.
- 1 2 "Russian scientists confirm 117th element". Sputnik. 2012-06-25. Retrieved 2012-07-05.
- ↑ Chow, D. (2014-05-01). "New Super-Heavy Element 117 Confirmed by Scientists". LiveScience. Retrieved 2014-05-02.
- ↑ IUPAC (2015). "Discovery and Assignment of Elements with Atomic Numbers 113, 115, 117 and 118". Retrieved 2016-01-04.
- ↑ Chatt, J. (1979). "Recommendations for the Naming of Elements of Atomic Numbers Greater than 100". Pure Appl. Chem. 51 (2): 381–384. doi:10.1351/pac197951020381.
- ↑ Koppenol, W. H. (2002). "Naming of new elements (IUPAC Recommendations 2002)" (PDF). Pure and Applied Chemistry. 74 (5): 787–791. doi:10.1351/pac200274050787.
- ↑ "How to name new chemical elements (IUPAC Recommendations 2016)". Pure and Applied Chemistry. doi:10.1515/pac-2015-0802.
- ↑ Glanz, J. (2010). "Scientists Discover Heavy New Element". Oregon State University, Department of Chemistry. Retrieved 2016-01-05.
- ↑ "IUPAC Is Naming The Four New Elements Nihonium, Moscovium, Tennessine, And Oganesson". IUPAC. 2016-06-08. Retrieved 2016-06-08.
- ↑ "IUPAC Announces the Names of the Elements 113, 115, 117, and 118 - IUPAC | International Union of Pure and Applied Chemistry". IUPAC | International Union of Pure and Applied Chemistry. 2016-11-30. Retrieved 2016-11-30.
- ↑ de Marcillac, P.; Coron, N.; Dambier, G.; et al. (2003). "Experimental detection of α-particles from the radioactive decay of natural bismuth". Nature. 422 (6934): 876–878. Bibcode:2003Natur.422..876D. doi:10.1038/nature01541. PMID 12712201.
- ↑ Considine, G. D.; Kulik, Peter H. (2002). Van Nostrand's scientific encyclopedia (9th ed.). Wiley-Interscience. ISBN 978-0-471-33230-5. OCLC 223349096.
- 1 2 "Element 117 is synthesized". JINR. 2010. Retrieved 2015-06-28.
- ↑ Zagrebaev, V.; Karpov, A.; Greiner, W. (2013). Future of superheavy element research: Which nuclei could be synthesized within the next few years? (PDF). Journal of Physics: Conference Series. 420. pp. 1–15. arXiv:1207.5700
 . doi:10.1088/1742-6596/420/1/012001. Retrieved 2013-08-20.
. doi:10.1088/1742-6596/420/1/012001. Retrieved 2013-08-20. - ↑ Zhao-Qing, F.; Gen-Ming, Jin; Ming-Hui, Huang; et al. (2007). "Possible Way to Synthesize Superheavy Element Z = 117". Chinese Physics Letters. 24 (9): 2551. arXiv:0708.0159
 . Bibcode:2007ChPhL..24.2551F. doi:10.1088/0256-307X/24/9/024.
. Bibcode:2007ChPhL..24.2551F. doi:10.1088/0256-307X/24/9/024. - ↑ Zhao-Qing, F.; Jina, Gen-Ming; Li, Jun-Qing; et al. (2009). "Production of heavy and superheavy nuclei in massive fusion reactions". Nuclear Physics A. 816: 33. arXiv:0803.1117
 . Bibcode:2009NuPhA.816...33F. doi:10.1016/j.nuclphysa.2008.11.003.
. Bibcode:2009NuPhA.816...33F. doi:10.1016/j.nuclphysa.2008.11.003. - ↑ Chowdhury, R. P.; Samanta, C.; Basu, D. N. (2008). "Search for long lived heaviest nuclei beyond the valley of stability". Physical Review C. 77 (4): 044603. arXiv:0802.3837
 . Bibcode:2008PhRvC..77d4603C. doi:10.1103/PhysRevC.77.044603.
. Bibcode:2008PhRvC..77d4603C. doi:10.1103/PhysRevC.77.044603. - ↑ Duarte, S. B.; Tavares, O. A. P.; Gonçalves, M.; et al. (September 2004). Half-life prediction for decay modes for superheavy nuclei (PDF) (Report). Notas de Física. Centro Brasileiro de Pesquisas Físicas. doi:10.1088/0954-3899/30/10/014. ISSN 0029-3865.
- ↑ Dhingra, A. (1999-12-01). The Sterling Dictionary Of Chemistry. Sterling Publishers Pvt. Ltd. p. 187. ISBN 978-81-7359-123-5. Retrieved 2015-07-23.
- ↑ Thayer 2010, pp. 63–64.
- 1 2 3 4 Fægri Jr., K.; Saue, T. (2001). "Diatomic molecules between very heavy elements of group 13 and group 17: A study of relativistic effects on bonding". The Journal of Chemical Physics. 115 (6): 2456. Bibcode:2001JChPh.115.2456F. doi:10.1063/1.1385366.
- ↑ Thayer 2010, pp. 63–67.
- ↑ Thayer 2010, p. 79.
- 1 2 Thayer 2010, p. 64.
- ↑ Pyykkö, P.; Atsumi, M. (2008-12-22). "Molecular Single-Bond Covalent Radii for Elements 1-118". Chemistry: A European Journal. 15: 186–197. doi:10.1002/chem.200800987.
- 1 2 Sharma, B. K. (2001). Nuclear and radiation chemistry (7th ed.). Krishna Prakashan Media. p. 147. ISBN 978-81-85842-63-9. Retrieved 2012-11-09.
- 1 2 Seaborg, G. T. (1994). Modern alchemy. World Scientific. p. 172. ISBN 981-02-1440-5.
- ↑ Takahashi, N. (2002). "Boiling points of the superheavy elements 117 and 118". Journal of Radioanalytical and Nuclear Chemistry. 251 (2): 299–301. doi:10.1023/A:1014880730282.
- ↑ Luig, H.; Keller, C.; Wolf, W.; et al. (2005). "Radionuclides". In Ullmann, F. Encyclopedia of industrial chemistry. Wiley-VCH. p. 23. doi:10.1002/14356007.a22_499. ISBN 978-3-527-30673-2.
- ↑ Punter, J.; Johnson, R.; Langfield, S. (2006). The essentials of GCSE OCR Additional science for specification B. Letts and Lonsdale. p. 36. ISBN 978-1-905129-73-7.
- ↑ Wiberg, E.; Wiberg, N.; Holleman, A. F. (2001). Inorganic chemistry. Academic Press. p. 423. ISBN 978-0-12-352651-9.
- ↑ Otozai, K.; Takahashi, N. (1982). "Estimation of the chemical form and the boiling point of elementary astatine by radiogas-chromatography". Radiochimica Acta. 31 (3‒4): 201‒203.
- ↑ Bader, R. F. W. "An introduction to the electronic structure of atoms and molecules". McMaster University. Retrieved 2008-01-18.
- 1 2 Pershina 2010, p. 504.
- ↑ Thayer 2010, p. 84.
- 1 2 3 Bae, Ch.; Han, Y.-K.; Lee, Yo. S. (2003-01-18). "Spin−Orbit and Relativistic Effects on Structures and Stabilities of Group 17 Fluorides EF3 (E = I, At, and Element 117): Relativity Induced Stability for the D3h Structure of (117)F3". The Journal of Physical Chemistry A. 107 (6): 852–858. doi:10.1021/jp026531m.
- 1 2 3 4 5 Han, Y.-K.; Bae, Cheolbeom; Son, Sang-Kil; et al. (2000). "Spin-orbit effects on the transactinide p-block element monohydrides MH (M=element 113-118)". Journal of Chemical Physics. 112 (6): 2684–2691. Bibcode:2000JChPh.112.2684H. doi:10.1063/1.480842.
- ↑ Stysziński 2010, pp. 144–146.
- ↑ Lide, D. R. (2003). "Section 9, Molecular Structure and Spectroscopy". CRC Handbook of Chemistry and Physics (84th ed.). CRC Press. pp. 9–45, 9–46. ISBN 978-0-8493-0484-2.
- ↑ Stysziński 2010, pp. 139–146.
- Bibliography
- Barysz, M.; Ishikawa, Y., eds. (2010). Relativistic methods for chemists. Springer Science+Business Media. ISBN 978-1-4020-9974-8.
- Thayer, J. S. (2010). Relativistic Effects and the Chemistry of the Heavier Main Group Elements. p. 63. doi:10.1007/978-1-4020-9975-5_2.
- Stysziński, J. (2010). Why do we need relativistic computational methods?. doi:10.1007/978-1-4020-9975-5_3.
- Pershina, V. (2010). Electronic structure and chemistry of the heaviest elements. doi:10.1007/978-1-4020-9975-5_11.
| Periodic table (Large cells) | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||||||||||||||||
| 1 | H | He | |||||||||||||||||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | |||||||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||||||||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |||||||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | |||||||||||||||
| 6 | Cs | Ba | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | |
| 7 | Fr | Ra | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
|
| |||||||||||||||||||||||||||||||||
