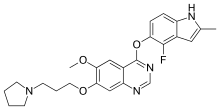
Cediranib
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | L01XE32 (WHO) |
| Pharmacokinetic data | |
| Biological half-life | 12 to 35 hours |
| Identifiers | |
| |
| CAS Number |
288383-20-0 |
| PubChem (CID) | 9933475 |
| IUPHAR/BPS | 5664 |
| ChemSpider |
8109103 |
| UNII |
NQU9IPY4K9 |
| ChEMBL |
CHEMBL491473 |
| ECHA InfoCard | 100.196.628 |
| Chemical and physical data | |
| Formula | C25H27FN4O3 |
| Molar mass | 450.505 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Cediranib (AZD-2171; tentative trade name Recentin) is a potent inhibitor of vascular endothelial growth factor (VEGF) receptor tyrosine kinases.[1][2][3]
The drug is being developed by AstraZeneca as a possible anti-cancer chemotherapeutic agent for oral administration.
Beginning in 2007, it underwent Phase I clinical trials for the treatment of non-small cell lung cancer, kidney cancer, and colorectal cancer in adults, as well as tumors of the central nervous system in children. Phase I trials of interactions with other drugs used in cancer treatment were also undertaken.
On February 27, 2008, AstraZeneca announced that the use of cediranib in non-small cell lung cancer will not progress into phase III after failing to meet its main goal. On 8 March 2010, AstraZeneca issued a press-release stating that cediranib had failed Phase III clinical trials for use in first-line metastatic colorectal cancer when it was compared clinically with the market-leader bevacizumab.[4] As of November 2012, it was being assessed in double-blind studies for the treatment of methylated Glioblastoma Multiforme at the University of Washington Medical Center at a 20 mg daily dose.
Combination trials
Findings from a federally funded, NCI-sponsored phase II clinical trial[5] presented at the 50th Annual Meeting of the American Society of Clinical Oncology (May 30 - June 3, 2014, Chicago, Ill; Abstract No: LBA5500),[6] show that the combination of two investigational oral drugs, olaparib (AZD-2281; AstraZeneca), a potential first-in-class poly ADP ribose polymerase or PARP inhibitor and cediranib (AZD-2171; AstraZeneca), an anti-angiogenesis drug, is significantly more active against recurrent, platinum chemotherapy-sensitive disease or ovarian cancer related to mutations in BRCA genes than olaparib alone.[7]
References
- ↑ Wedge SR, Kendrew J, Hennequin LF, et al. (May 2005). "AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer". Cancer Res. 65 (10): 4389–400. doi:10.1158/0008-5472.CAN-04-4409. PMID 15899831.
- ↑ Goss G, Shepherd FA, Laurie S, et al. (December 2008). "A phase I and pharmacokinetic study of daily oral cediranib, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with cisplatin and gemcitabine in patients with advanced non-small cell lung cancer: A study of the National Cancer Institute of Canada Clinical Trials Group". Eur. J. Cancer. 45 (5): 782–8. doi:10.1016/j.ejca.2008.10.022. PMID 19091548.
- ↑ Nikolinakos P, Heymach JV (June 2008). "The tyrosine kinase inhibitor cediranib for non-small cell lung cancer and other thoracic malignancies". J Thorac Oncol. 3 (6 Suppl 2): S131–4. doi:10.1097/JTO.0b013e318174e910. PMID 18520296.
- ↑ "AstraZeneca - RECENTIN did not meet primary endpoint in Horizon III study in metastatic colorectal cancer". Retrieved 17 March 2014.
- ↑ "Cediranib Maleate and Olaparib in Treating Patients With Recurrent Ovarian, Fallopian Tube, Peritoneal Cancer, or Triple-Negative Breast Cancer". Retrieved 12 June 2014.
- ↑ Liu J, Barry WT, Birrer MJ, et al. A randomized phase 2 trial comparing efficacy of the combination of the PARP inhibitor olaparib and the antiangiogenic cediranib against olaparib alone in recurrent platinum-sensitive ovarian cancer; J Clin Oncol 32:5s, 2014 (suppl; abstr LBA5500))
- ↑ Combination of Targeted Drugs May Significantly Increase Progression-Free Survival in Women with Recurrent Ovarian Cancer, Study Shows - Onco'Zine - The International Oncology Network; June 2, 2014