Canertinib
 | |
| Names | |
|---|---|
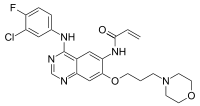
| IUPAC name
N-{4-[(3-Chloro-4-fluorophenyl)amino]-7-[3-(morpholin-4-yl)propoxy]quinazolin-6-yl}prop-2-enamide | |
| Other names
CI-1033; PD-183805 | |
| Identifiers | |
| 267243-28-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:61399 |
| ChEMBL | ChEMBL31965 ChEMBL545315 |
| ChemSpider | 137741 |
| 5675 | |
| PubChem | 156414 |
| UNII | C78W1K5ASF |
| |
| |
| Properties | |
| C24H25ClFN5O3 | |
| Molar mass | 485.94 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Canertinib (CI-1033) is an experimental drug candidate for the treatment of cancer. It is an irreversible tyrosine-kinase inhibitor with activity against EGFR (IC50 0.8 nM), HER-2 (IC50 19 nM) and ErbB-4 (IC50 7 nM).[1][2]
Drug Interactions: Canertinib has been reported as a substrate for OATP1B3. Interaction of canertinib with OATP1B3 may alter its hepatic disposition and can lead to transporter mediated drug-drug interactions.[3] Also, canertinib is not an inhibitor of OATP-1B1 or OATP-1B3 transporter.[4]
References
- ↑ Smaill, JB; Rewcastle, GW; Loo, JA; Greis, KD; Chan, OH; Reyner, EL; Lipka, E; Showalter, HD; et al. (2000). "Tyrosine kinase inhibitors. 17. Irreversible inhibitors of the epidermal growth factor receptor: 4-(phenylamino)quinazoline- and 4-(phenylamino)pyrido3,2-dpyrimidine-6-acrylamides bearing additional solubilizing functions". Journal of Medicinal Chemistry. 43 (7): 1380–97. doi:10.1021/jm990482t. PMID 10753475.
- ↑ CI-1033 (Canertinib), Selleck Chemicals
- ↑ Khurana V, Minocha M, Pal D, Mitra AK (March 2014). "Role of OATP-1B1 and/or OATP-1B3 in hepatic disposition of tyrosine kinase inhibitors.". Drug Metabol Drug Interact. 29 (3): 1–11. doi:10.1515/dmdi-2013-0062. PMID 24643910.
- ↑ Khurana V, Minocha M, Pal D, Mitra AK (May 2014). "Inhibition of OATP-1B1 and OATP-1B3 by tyrosine kinase inhibitors.". Drug Metabol Drug Interact. 29 (4): 1–11. doi:10.1515/dmdi-2014-0014. PMID 24807167.
This article is issued from Wikipedia - version of the 8/5/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.