Viologen
Viologens are toxic bipyridinium derivatives of 4,4'-bipyridyl.[1] The name is because this class of compounds is easily reduced to the radical mono cation, which is colored intensely blue.
Possibly the best-known viologen is paraquat, which is one of the world's most widely used herbicides.
Electrochemistry
Viologens are used for electrochromic systems because of their ability to change color reversibly many times upon reduction and oxidation.
In an experimental electrolysis arrangement, viologen in solution with sodium sulfate can be reduced at a cathode with simultaneous formation of hydrogen gas. Oxygen generated at the anode is capable of oxidizing the radical ion back to the viologen.

Further reduction yields a yellow quinoid compound. Diquaternary derivatives of 2,2'-bipyridyl give a green radical anion.
In extended viologens, conjugated oligomers such as based on aryl, ethylene, and thiophene units are inserted between the pyridine units.[2] The bipolaron di-octyl bis(4-pyridyl)biphenyl viologen 2 in scheme 2 can be reduced by sodium amalgam in DMF to the neutral viologen 3.
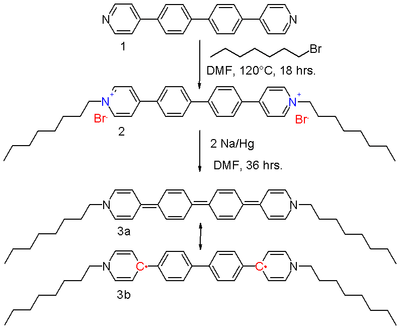
The resonance structures of the quinoid 3a and the biradical 3b contribute equally to the hybrid structure. The driving force for the contributing 3b is the restoration of aromaticity with the biphenyl unit. It has been established using X-ray crystallography that the molecule is, in effect, coplanar with slight nitrogen pyramidalization, and that the central carbon bonds are longer (144 pm) than what would be expected for a double bond (136 pm). Further research shows that the diradical exists as a mixture of triplets and singlets, although an ESR signal is absent. In this sense, the molecule resembles Tschischibabin's hydrocarbon, discovered during 1907. It also shares with this molecule a blue color in solution, and a metallic-green color as crystals.
Compound 3 is a very strong reducing agent, with a redox potential of −1.48 V, again because aromaticity is restored. The compound is also a liquid crystal with multiple liquid crystal phases in the melt as a result of the molecule's structure with a flat and rigid core and flexible linear alkyl branches.
Viologen catalysts have been reported to have the potential to oxidize glucose and other carbohydrates catalytically in a mildly alkaline solution, which makes direct carbohydrate fuel cells possible.[3]
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "viologens".
- ↑ W. W. Porter, T. P. Vaid and A. L. Rheingold (2005). "Synthesis and Characterization of a Highly Reducing Neutral "Extended Viologen" and the Isostructural Hydrocarbon 4,4' '-Di-n-octyl-p-quaterphenyl". J. Am. Chem. Soc. 127 (47): 16559–16566. doi:10.1021/ja053084q. PMID 16305245.
- ↑ Dean R. Wheeler; Joseph Nichols; Dane Hansen; Merritt Andrus; Sang Choi & Gerald D. Watt (2009). "Viologen Catalysts for a Direct Carbohydrate Fuel Cell". J. Electrochem. Soc. 156 (10): B1201–B1207. doi:10.1149/1.3183815.