Aryl
This article is about the aryl organic functional group. For the fleshy covering of certain seeds, see aril.
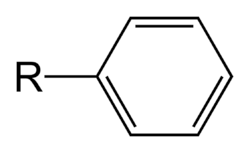
A phenyl group is the simplest aryl group, here bonded to an "R" group.
In the context of organic molecules, aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, be it phenyl, naphthyl, thienyl, indolyl, etc. (see IUPAC nomenclature).[1] "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as a placeholder for the aryl group in chemical structure diagrams.
A simple aryl group is phenyl, C6H5; it is derived from benzene. The tolyl group, CH3C6H4, is derived from toluene (methylbenzene). The xylyl group, (CH3)2C6H3, is derived from xylene (dimethylbenzene), while the naphthyl group, C10H7, is derived from naphthalene.
Arylation is a chemical process in which an aryl group is attached to a substituent.
See also
- Alkyl
- Aryl hydrocarbon receptor, a bodily target for dioxins[2]
- Arene compound
References
- ↑ http://goldbook.iupac.org/A00464.html
- ↑ Bock KW, Köhle C (2006). "Ah receptor: dioxin-mediated toxic responses as hints to deregulated physiologic functions". Biochem. Pharmacol. 72 (4): 393–404. doi:10.1016/j.bcp.2006.01.017. PMID 16545780.
This article is issued from Wikipedia - version of the 6/29/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.