Phosphofructokinase
| Phosphofructokinase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Ppfruckinase | ||||||||
| Pfam | PF00365 | ||||||||
| InterPro | IPR000023 | ||||||||
| PROSITE | PDOC00336 | ||||||||
| |||||||||
Phosphofructokinase is a kinase enzyme that phosphorylates fructose 6-phosphate in glycolysis.
The enzyme-catalysed transfer of a phosphoryl group from ATP is an important reaction in a wide variety of biological processes.[1] One enzyme that utilizes this reaction is phosphofructokinase (PFK), which catalyses the phosphorylation of fructose-6-phosphate to fructose-1,6- bisphosphate, a key regulatory step in the glycolytic pathway.[2][3] It is allosterically inhibited by ATP and allosterically activated by AMP, thus indicating the cell's energetic needs when it undergoes the glycolytic pathway.[4] PFK exists as a homotetramer in bacteria and mammals (where each monomer possesses 2 similar domains) and as an octomer in yeast (where there are 4 alpha- (PFK1) and 4 beta-chains (PFK2), the latter, like the mammalian monomers, possessing 2 similar domains[3]). This protein may use the morpheein model of allosteric regulation.[5]
PFK is about 300 amino acids in length, and structural studies of the bacterial enzyme have shown it comprises two similar (alpha/beta) lobes: one involved in ATP binding and the other housing both the substrate-binding site and the allosteric site (a regulatory binding site distinct from the active site, but that affects enzyme activity). The identical tetramer subunits adopt 2 different conformations: in a 'closed' state, the bound magnesium ion bridges the phosphoryl groups of the enzyme products (ADP and fructose-1,6- bisphosphate); and in an 'open' state, the magnesium ion binds only the ADP,[6] as the 2 products are now further apart. These conformations are thought to be successive stages of a reaction pathway that requires subunit closure to bring the 2 molecules sufficiently close to react.[6]
Deficiency in PFK leads to glycogenosis type VII (Tarui's disease), an autosomal recessive disorder characterised by severe nausea, vomiting, muscle cramps and myoglobinuria in response to bursts of intense or vigorous exercise.[3] Sufferers are usually able to lead a reasonably ordinary life by learning to adjust activity levels.[3]
Regulation
There are two types of the enzyme:
| Type | Synonyms | EC number | Substrate | Product | Subunit genes |
|---|---|---|---|---|---|
| Phosphofructokinase 1 | 6-phosphofructokinase phosphohexokinase | EC 2.7.1.11 | 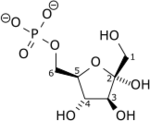 Fructose 6-phosphate | 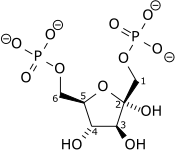 Fructose-1,6-bisphosphate | PFKL, PFKM, PFKP |
| Phosphofructokinase 2 | 6-phosphofructo-2-kinase | EC 2.7.1.105 | 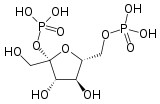 Fructose-2,6-bisphosphate | PFKFB1, PFKFB2, PFKFB3, PFKFB4 |
See also
References
- ↑ Evans PR, Hellinga HW (1987). "Mutations in the active site of Escherichia coli phosphofructokinase". Nature. 327 (6121): 437–439. doi:10.1038/327437a0. PMID 2953977.
- ↑ Wegener G, Krause U (2002). "Different modes of activating phosphofructokinase, a key regulatory enzyme of glycolysis, in working vertebrate muscle". Biochem. Soc. Trans. 30 (2): 264–270. doi:10.1042/bst0300264. PMID 12023862.
- 1 2 3 4 Raben N, Exelbert R, Spiegel R, Sherman JB, Nakajima H, Plotz P, Heinisch J (1995). "Functional expression of human mutant phosphofructokinase in yeast: genetic defects in French Canadian and Swiss patients with phosphofructokinase deficiency". Am. J. Hum. Genet. 56 (1): 131–141. PMC 1801305
 . PMID 7825568.
. PMID 7825568. - ↑ Garrett, Reginald; Grisham, Reginald (2012). Biochemistry. Cengage Learning. p. 585. ISBN 978-1133106296.
- ↑ T. Selwood; E. K. Jaffe. (2011). "Dynamic dissociating homo-oligomers and the control of protein function.". Arch. Biochem. Biophys. 519 (2): 131–43. doi:10.1016/j.abb.2011.11.020. PMC 3298769
 . PMID 22182754.
. PMID 22182754. - 1 2 Shirakihara Y, Evans PR (1988). "Crystal structure of the complex of phosphofructokinase from Escherichia coli with its reaction products". J. Mol. Biol. 204 (4): 973–994. doi:10.1016/0022-2836(88)90056-3. PMID 2975709.
External links
- Phosphofructokinases at the US National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the public domain Pfam and InterPro IPR000023