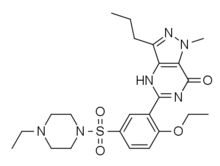
Homosildenafil
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | 642928-07-2 |
| PubChem (CID) | 24756844 |
| ChemSpider | 22547032 |
| UNII |
0Z3JH0S0QK |
| Chemical and physical data | |
| Formula | C23H32N6O4S |
| Molar mass | 488.6 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Homosildenafil (also known as methyl-sildenafil) is a synthetic drug which acts as a phosphodiesterase inhibitor.[1] It is an analog of sildenafil and vardenafil. Homosildenafil was first identified as an adulterant in sex enhancement products in 2003 and was more recently detected in dietary supplements.[2][3][4][5]
Homosildenafil has 35% the PDE5 inhibition activity of sildenafil itself with similar selectivity.[1]
Sildenfil is mainly metabolized by the microsomal isozymes CYP3A4 with secondary metabolism by CYP2C9. The major active metabolite is N-desmethylsildenafil. The plasma level of the equivalent homosildenafil metabolite reaches 40% of sildenafil's bioavailability. The N-desmethyl metabolite is further metabolized, with a half-life of 4 hours.[6][7][8][9]
See also
References
- 1 2 Jackie D Corbin; Alfreda Beasley; Mitsi A Blount; Sharron H Francis (November 2004). "Vardenafil: structural basis for higher potency over sildenafil in inhibiting cGMP-specific phosphodiesterase-5 (PDE5)". Neurochemistry International. 45 (6): 859–863. doi:10.1016/j.neuint.2004.03.016. PMID 15312980.
- ↑ M.H. Shin; M.K. Hong; W.S. Kim; Y.J. Lee; Y.C. Jeoung (November 2003). "Identification of a new analogue of sildenafil added illegally to a functional food marketed for penile erectile dysfunction". Food Additives & Contaminants. 20 (9): 793–796. doi:10.1080/0265203031000121455. PMID 13129773.
- ↑ Connie M. Gryniewicz; John C. Reepmeyer; John F. Kauffman; Lucinda F. Buhse (April 2009). "Detection of undeclared erectile dysfunction drugs and analogues in dietary supplements by ion mobility spectrometry". Journal of Pharmaceutical and Biomedical Analysis. 49 (5): 601–606. doi:10.1016/j.jpba.2008.12.002. PMID 19150190.
- ↑ "Advisory - "Forta for Men" sex enhancement product recalled; contains undeclared drug". PR Newswire. 2014. Retrieved 28 April 2015.
- ↑ Peng Zoua; Sharon Sze-Yin Ohb; Peiling Houa; Min-Yong Lowb; Hwee-Ling Koh (February 2006). "Simultaneous determination of synthetic phosphodiesterase-5 inhibitors found in a dietary supplement and pre-mixed bulk powders for dietary supplements using high-performance liquid chromatography with diode array detection and liquid chromatography–electrospray ionization tandem mass spectrometry". Journal of Chromatography A. 1104 (1–2): 113–122. doi:10.1016/j.chroma.2005.11.103. PMID 16364350.
- ↑ Tejada Inigo Saenz De; Kenneth M Ferguson; John S Whitaker (2002). "Daily treatment for erectile dysfunction using a pde5 inhibitor". Retrieved 27 April 2015.
- ↑ Ivan M. Borrello; Paolo Serafini; Kimberly A. Noonan; Vincenzo Bronte (2009). "Pde5 inhibitor compositions and methods for immunotherapy". Retrieved 27 April 2015.
- ↑ Ing-Jun Chen (2013). "Processes for preparing amine salts of sildenafil-analogues and use thereof". Retrieved 27 April 2015.
- ↑ 杨 许; 郭杰标; 黄志兵 (2009). "Preparation method for monoclonal antibody specifically binding vardenafil and analog thereof". Retrieved 27 April 2015.