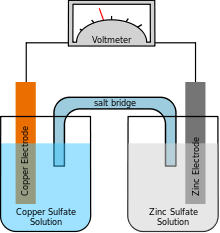
Comparison of battery types
Common characteristics
| Cell chemistry | Also known as | Electrode | Rechargeable | Commercialized | Voltage | Energy density | Specific power | Cost† | Discharge efficiency | Self-discharge rate | Shelf life | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anode | Cathode | Cutoff | Nominal | 100% SOC | by mass | by volume | |||||||||
| year | V | V | V | MJ/kg (Wh/kg) |
MJ/L (Wh/L) |
W/kg | Wh/$ ($/kWh) |
% | %/month | years | |||||
| Lead-acid | SLA VRLA |
Lead | Lead dioxide | Yes | 1881[1] | 1.75[2] | 2.1[2] | 2.23–2.32[2] | 0.11–0.14 (30–40)[2] |
0.22–0.27 (60–75)[2] |
180[2] | 6.99–17.98 (56–143)[2] |
50–92[2] | 3–20[2] | |
| Zinc-carbon | Carbon-zinc | Zinc | Carbon | No | 1898[3] | 0.75–0.9[3] | 1.5[3] | 0.13 (36)[3] |
0.33 (92)[3] |
10–27[3] | 3.2 (313)[3] |
50–60[3] | 0.32[3] | 3–5[4] | |
| Zinc-air | PR | Oxygen | No | 1932[5] | 0.9[5] | 1.45–1.65[5] | 1.59 (442)[5] |
6.02 (1,673)[5] |
100[5] | 2.8 (358)[5] |
60–70[5] | 0.17[5] | 3[5] | ||
| Mercury oxide-zinc | Mercuric oxide Mercury cell |
Mercuric oxide | No | 1942–[6] 1996[7] | 0.9[8] | 1.35[8] | 0.36–0.44 (99–123)[8] |
1.1–1.8 (300–500)[8] |
2[6] | ||||||
| Alkaline | Zn/MnO 2 LR |
Manganese (IV) oxide | No | 1949[9] | 0.9[10] | 1.5[11] | 1.6[10] | 0.31–0.68 (85–190)[12] |
0.90–1.56 (250–434)[12] |
50[12] | 0.5 (2003)[12] |
45–85[12] | 0.17[12] | 5–10[4] | |
| Rechargeable alkaline | RAM | Yes | 1992[13] | 0.9[14] | 1.57[14] | 1.6[14] | 1 ! <1[13] | ||||||||
| Silver-oxide | SR | Silver oxide | No | 1960[15] | 1.2[16] | 1.55[16] | 1.6[17] | 0.47 (130)[17] |
1.8 (500)[17] |
||||||
| Nickel-zinc | NiZn | Nickel oxide hydroxide | Yes | 2009[13] | 0.9[13] | 1.65[13] | 1.85[13] | 13[13] | |||||||
| Nickel-iron | NiFe | Iron | Yes | 1901[18] | 4.5[19] | 6[19] | 7.2[19] | 0.07–0.09 (19–25)[20] |
0.45 (125)[21] |
100 | 4.25–5.67 (176–235)[1] |
20–30 | 30–[22] 50[23][24] | ||
| Nickel-cadmium | NiCd NiCad |
Cadmium | Yes | 1960 !c. 1960[25] | 0.9–1.05[26] | 1.2[27] | 1.3[26] | 0.11 (30)[27] |
0.36 (100)[27] |
150–200[28] | 10[13] | ||||
| Nickel-hydrogen | NiH 2 Ni-H 2 |
Hydrogen | Yes | 1975[29] | 1.0[30] | 1.55[28] | 0.16–0.23 (45–65)[28] |
0.22 (60)[31] |
150–200[28] | 5[31] | |||||
| Nickel-metal hydride | NiMH Ni-MH |
Metal hydride | Yes | 1990[1] | 0.9–1.05[26] | 1.2[11] | 1.3[26] | 0.36 (100)[11] |
1.44 (401)[32] |
250–1000 | 3.4 (294)[1] |
30[33] | |||
| Low self-discharge nickel-metal hydride | LSD NiMH | Yes | 2005[34] | 0.9–1.05[26] | 1.2 | 1.3[26] | 0.34 (95)[35] |
1.27 (353)[36] |
250–1000 | 0.42[33] | |||||
| Lithium-manganese dioxide | Lithium Li-MnO 2 CR Li-Mn |
Lithium | Manganese dioxide | No | 1976[37] | 2[38] | 3[11] | 0.54–1.19 (150–330)[39] |
1.1–2.6 (300–710)[39] |
250–400[39] | 1 | 5-10[39] | |||
| Lithium-carbon monofluoride | Li-(CF) x BR |
Carbon monofluoride | No | 1976[37] | 2[40] | 3[40] | 0.94–2.81 (260–780)[39] |
1.58–5.32 (440–1,478)[39] |
50–80[39] | 0.2–0.3[41] | 15[39] | ||||
| Lithium-iron disulfide | Li-FeS 2 FR |
Iron disulfide | No | 1989[42] | 0.9[42] | 1.5[42] | 1.8[42] | 1.07 (297)[42] |
2.1 (580)[43] |
0.05[42] | 10–20[42] | ||||
| Lithium cobalt oxide | LiCoO 2 ICR LCO Li‑cobalt[44] |
Graphite‡ | Lithium cobalt oxide | Yes | 1991[45] | 2.5[46] | 3.7[47] | 4.2[46] | 0.70 (195)[47] |
2.0 (560)[47] |
2.83 (353)[1] |
||||
| Lithium iron phosphate | LiFePO 4 IFR LFP Li‑phosphate[44] |
Lithium iron phosphate | Yes | 1996[48] | 2[46] | 3.2[47] | 3.65[46] | 0.32–0.47 (90–130)[47] |
1.20 (333)[47] |
200 [49] | 4.5 | ||||
| Lithium manganese oxide | LiMn 2O 4 IMR LMO Li‑manganese[44] |
Lithium manganese oxide | Yes | 1999[1] | 2.5[50] | 3.9[47] | 4.2[50] | 0.54 (150)[47] |
1.5 (420)[47] |
2.83 (353)[1] |
|||||
| Lithium nickel cobalt aluminum oxide | LiNiCoAlO 2 NCA Li‑aluminum[44] |
Lithium nickel cobalt aluminum oxide | Yes | 1999 | 3.0[51] | 3.6[47] | 4.3[51] | 0.79 (220)[47] |
2.2 (600)[47] |
||||||
| Lithium nickel manganese cobalt oxide | LiNiMnCoO 2 INR NMC[44] NCM[47] |
Lithium nickel manganese cobalt oxide | Yes | 2008[52] | 2.5[46] | 3.6[47] | 4.2[46] | 0.74 (205)[47] |
2.1 (580)[47] |
||||||
^† Cost in USD, adjusted for inflation.
^‡ Typical. See Lithium-ion battery § Negative electrode for alternative electrode materials.
Rechargeable characteristics
| Cell chemistry | Charge efficiency | Cycle durability |
|---|---|---|
| % | # cycles | |
| Lead-acid | 50–92[2] | 500 typical, 800 max[2] |
| Rechargeable alkaline | 5-100[13] | |
| Nickel-zinc | 100 to 50% capacity[13] | |
| Nickel-iron | 65–80 | 5000 |
| Nickel-cadmium | 500[25] | |
| Nickel-hydrogen | 20,000[31] | |
| Nickel-metal hydride | 66 | 300–800[13] |
| Low self-discharge nickel-metal hydride battery | 500-1500[13] | |
| Lithium cobalt oxide | 500–1000 | |
| Lithium iron phosphate | 1000–2000 | |
| Lithium manganese oxide | 300–700 | |
| Lithium nickel cobalt aluminum oxide | 1000-1500[53] | |
| Lithium nickel manganese cobalt oxide | 5000[53] |
Thermal runaway
Under certain conditions, some battery chemistries are at risk of thermal runaway, leading to cell rupture or combustion. As thermal runaway is determined not only by cell chemistry but also cell size, cell design, and charge[54] only the worst-case values are reflected here.
| Cell chemistry | Overcharge | Overheat | ||
|---|---|---|---|---|
| Onset | Onset | Runaway | Peak | |
| SOC% | °C | °C | °C/min | |
| Lithium cobalt oxide | 150[54] | 165[54] | 190[54] | 440[54] |
| Lithium iron phosphate | 100[54] | 220[54] | 240[54] | 21[54] |
| Lithium manganese oxide | 110[54] | 210[54] | 240[54] | 100+[54] |
| Lithium nickel cobalt aluminum oxide | 125[54] | 140[54] | 195[54] | 260[54] |
| Lithium nickel manganese cobalt oxide | 170[54] | 160[54] | 230[54] | 100+[54] |
See also
- List of battery sizes
- List of battery types
- Battery nomenclature
- Experimental rechargeable battery types
References
- 1 2 3 4 5 6 7 "mpoweruk.com: Accumulator and battery comparisons (pdf)" (PDF). Retrieved 2016-02-28.
- 1 2 3 4 5 6 7 8 9 10 11 "All About Batteries, Part 3: Lead-Acid Batteries". Retrieved 2016-02-26.
- 1 2 3 4 5 6 7 8 9 "All About Batteries, Part 5: Carbon Zinc Batteries". Retrieved 2016-02-26.
- 1 2 "Energizer Non-Rechargeable Batteries: Frequently Asked Questions" (PDF). Retrieved 2016-02-26.
- 1 2 3 4 5 6 7 8 9 10 "All About Batteries, Part 6: Zinc-Air". Retrieved 2016-03-01.
- 1 2 Narayan, R.; Viswanathan, B. (1998). Chemical And Electrochemical Energy Systems. Universities Press. p. 92.
- ↑ "Mercury Use in Batteries". Retrieved 2016-03-01.
- 1 2 3 4 Crompton, Thomas Roy (2000). Batteries Reference Book. Newnes. Retrieved 2016-03-01.
- ↑ Herbert, W. S. "The Alkaline Manganese Dioxide Dry Cell" (PDF). Journal of the Electrochemical Society (August 1952). Retrieved 2016-03-01.
- 1 2 "Alkaline Manganese Dioxide Handbook and Application Manual" (PDF). Retrieved 2016-03-01.
- 1 2 3 4 "Primary and Rechargeable Battery Chemistries with Energy Density". Retrieved 2016-02-26.
- 1 2 3 4 5 6 "All About Batteries, Part 4: Alkaline Batteries". Retrieved 2016-02-26.
- 1 2 3 4 5 6 7 8 9 10 11 12 "Rechargeable Batteries — compared and explained in detail". Retrieved 2016-02-28.
- 1 2 3 "Data Sheet of Pure Energy XL Rechargeable Alkaline Cells" (PDF). Retrieved 2016-03-01.
- ↑ "The history of the battery: 2) Primary batteries". Retrieved 2016-03-01.
- 1 2 "Silver Primary Cells & Batteries" (PDF). Archived from the original (PDF) on December 15, 2009. Retrieved 2016-03-01.
- 1 2 3 "ProCell Silver Oxide battery chemistry". Duracell. Archived from the original on 2009-12-20. Retrieved 2009-04-21.
- ↑ "Edison's non-toxic nickel-iron battery revived in ultrafast form". Retrieved 2016-02-28.
- 1 2 3 "Nickel-Iron Power" (PDF). Retrieved 2016-03-01.
- ↑ "Energy Density from NREL Testing by Iron Edison" (PDF). Retrieved 2016-02-26.
- ↑ Jha, A.R. (2012-06-05). Next-Generation Batteries and Fuel Cells for Commercial, Military, and Space Applications. p. 28. ISBN 1439850666.
- ↑ Mpower: Nickel Iron Batteries
- ↑ A description of the Chinese nickel–iron battery from BeUtilityFree
- ↑ "Nickel Iron Battery Frequently Asked Questions" BeUtilityFree
- 1 2 "Nickel Cadmium Batteries". Electropaedia. Woodbank Communications. Retrieved 2016-02-29.
- 1 2 3 4 5 6 "Testing NiCd and NiMH Batteries". Retrieved 2016-03-01.
- 1 2 3 "Getting to know more about batteries". Retrieved 2016-02-26.
- 1 2 3 4 "Optimization of spacecraft electrical power subsystems" (PDF). Retrieved 2016-02-29.
- ↑ "Nickel-Hydrogen Battery Technology—Development and Status" (PDF). Archived from the original (PDF) on 2009-03-18. Retrieved 2012-08-29.
- ↑ Thaller, Lawrence H.; Zimmerman, Albert H. (2003). Nickel-hydrogen Life Cycle Testing. AIAA.
- 1 2 3 Spacecraft Power Systems Pag.9
- ↑ "Ansmann AA – NiMH 2700mAh datasheet" (PDF). Retrieved 2016-03-02.
- 1 2 "AA Battery Considerations". Retrieved 2016-03-01.
- ↑ "General Description". Eneloop.info. Sanyo. Archived from the original on 2012-09-02. Retrieved 2015-08-06.
- ↑ "Metero Webinar 2". Retrieved 2016-03-02.
- ↑ "SANYO new Eneloop Batteries Remains Energy Longer" (PDF). Retrieved 2016-03-02.
- 1 2 Dyer, Chris K; Moseley, Patrick T; Ogumi, Zempachi; Rand, David A. J.; Scrosati, Bruno (2013). Encyclopedia of Electrochemical Power Sources. Newnes. p. 561. ISBN 0444527451. Retrieved 2016-03-03.
- ↑ "Lithium Manganese Dioxide Batteries CR2430" (PDF). Retrieved 2016-03-01.
- 1 2 3 4 5 6 7 8 "Li/CFx Batteries: The Renaissance" (PDF). Retrieved 2016-03-03.
- 1 2 "Chapter 1 Overview - Industrial Devices and Solutions" (PDF). Retrieved 2016-03-03.
- ↑ "Lithium Carbon-monofluoride (BR) Coin Cells and FB Encapsulated Lithium Coin Cells". Retrieved 2016-03-03.
- 1 2 3 4 5 6 7 "Lithium Iron Disulfide Handbook and Application Manual" (PDF). Retrieved 2016-03-03.
- ↑ "Energizer's Lithium Iron Disulfide – The best of all worlds for the most demanding applications" (PDF). Retrieved 2016-03-03.
- 1 2 3 4 5 "Battery chemistry FINALLY explained". Retrieved 2016-02-26.
- ↑ "Hooked on lithium". Retrieved 2016-02-26.
- 1 2 3 4 5 6 "Comparison Common Lithium Technologies" (PDF). Retrieved 2016-03-01.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 "Lithium Battery Technologies". Retrieved 2016-02-26.
- ↑ "LiFePO
4: A Novel Cathode Material for Rechargeable Batteries", A.K. Padhi, K.S. Nanjundaswamy, J.B. Goodenough, Electrochemical Society Meeting Abstracts, 96-1, May, 1996, pp 73 - ↑ https://www.victronenergy.nl/upload/documents/Datasheet-12,8-Volt-lithium-iron-phosphate-batteries-EN.pdf
- 1 2 "Lithium-ion Battery Overview" (PDF). Lighting Global (May 2012, Issue 10). Retrieved 2016-03-01.
- 1 2 "Lithium nickel cobalt aluminium oxide". Retrieved 2016-03-01.
- ↑ "Battery Technology". Retrieved 2016-02-26.
- 1 2 "Why Tesla's grid batteries will use two different chemistries". Retrieved 2016-03-02.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Doughty, Dan; Roth, E. Peter. "A General Discussion of Li Ion Battery Safety" (PDF). The Electrochemical Society Interface (Summer 2012). Retrieved 2016-02-27.
This article is issued from Wikipedia - version of the 11/21/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.