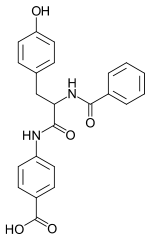
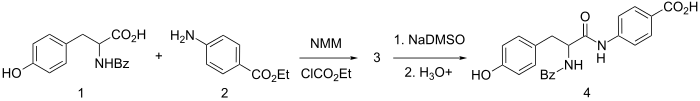Bentiromide
 | |
| Names | |
|---|---|
| IUPAC name
4-[2-benzamido-3- (4-hydroxyphenyl)- propanoyl] aminobenzoic acid | |
| Other names
(S)-4-((2-(benzoylamino)-3-(4-hydroxyphenyl) -1-oxopropyl)amino)benzoic acid (S)-p-(α-benzamido-p-hydroxyhydrocinnamamido) benzoic acid Benzoyltyrosyl-p-aminobenzoic acid (Btpaba)Chymex N-benzoyl-L-tyrosyl-p-aminobenzoic acid P-((N-benzoyl-L-tyrosin)amido)benzoic acid Chymex (trade name) | |
| Identifiers | |
| 37106-97-1 | |
| 3D model (Jmol) | Interactive image |
| Abbreviations | Btpaba |
| ChEBI | CHEBI:31263 |
| ChEMBL | ChEMBL1200368 |
| ChemSpider | 5329364 |
| DrugBank | DB00522 |
| ECHA InfoCard | 100.048.484 |
| EC Number | 253-349-8 |
| PubChem | 6957673 |
| UNII | 239IF5W61J |
| |
| |
| Properties | |
| C23H20N2O5 | |
| Molar mass | 404.4153 g/mol |
| Pharmacology | |
| V04CK03 (WHO) | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Bentiromide is a peptide used as a screening test for exocrine pancreatic insufficiency and to monitor the adequacy of supplemental pancreatic therapy. It is given by mouth as a noninvasive test. The amount of 4-aminobenzoic acid and its metabolites excreted in the urine is taken as a measure of the chymotrypsin-secreting activity of the pancreas. Headache and gastrointestinal disturbances have been reported in patients taking bentiromide. Bentiromide is not available in the United States or Canada (it was withdrawn in the U.S. in October 1996.)
Data
- XLogP=3.201
- tautomers=12
- H_bond_donor=4
- H_bond_acceptor=5
Synthesis
Synthetic chymotrypsin-labile peptide, used in diagnosis of exocrine pancreatic disease.
It is synthesized by amide formation between ethyl p-aminobenzoate and N-benzoyl-tyrosine using N-methyl-morpholine and ethyl chlorocarbonate for activation. The resulting L-amide is selectively hydrolyzed by sequential use of dimsyl sodium (NaDMSO) and dilute acid to give bentiromide (4).
See also
References
- ↑ Bentiromide – Compound Summary, PubChem.
- ↑ P. L. De Benneville, N. J. Greenberger, DE 2156835; eidem, U.S. Patent 3,801,562 (1972, 1974 both to Rohm & Haas).
- ↑ Debenneville, Peter L.; Godfrey, William J.; Sims, Homer J.; Imondi, Anthony R. (1972). "New substrates for a pancreatic exocrine function test". Journal of Medicinal Chemistry. 15 (11): 1098. doi:10.1021/jm00281a002. PMID 4654657.