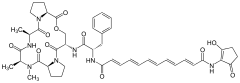
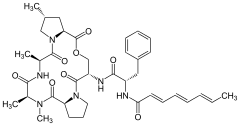
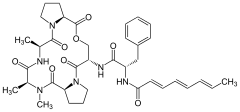
Acyldepsipeptide antibiotics
Acyldepsipeptide or cyclic acyldepsipeptide (ADEP) is a class of potential antibiotics first isolated from bacteria. Natural ADEPs were originally found as products of aerobic fermentation in Streptomyces hawaiiensis, A54556A and B,[1] and in the culture broth of Streptomyces species, enopeptin A and B.[2] ADEPs are of great interest in drug development due to their antibiotic properties and thus are being modified in attempt to achieve greater antimicrobial activity.[3][4]
The potential role of ADEPs in combating antibiotic drug resistance is postulated due to their novel mode of action that other antibiotics are not known to use, activation of casein lytic protease (ClpP) which is an important bacterial protease.[5] Most antibiotics work through inhibitory processes to establish cell death, while ADEPs actually work through activation of the protease complex to cause uncontrolled protease degradation, inhibition of cell division, and subsequent cell death.[3][4][6] They largely affect Gram-positive bacteria[4] and could be of great use to target antibiotic resistant microbes such as methicillin-resistant Staphylococcus aureus (MRSA), penicillin-resistant Streptococcus pneumonia (PRSP), Mycobacterium tuberculosis, and others.[3][4] Despite the potential use of ADEP, possible resistance has been examined in certain species.[7]
| Chemical structures of natural ADEPs | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Mechanism
The peptidase ClpP is highly conserved throughout organisms and is tightly regulated.[4] Without activation, ClpP in normal conditions can degrade short peptides that freely diffuse into its inner degradation chamber.[8] However, for folded proteins, unfolded proteins, and long peptides, ClpP must be activated by a protein in the family of ATPase associated with diverse cellular activities (AAA proteins), such as ClpA, ClpX, or ClpC.[8] These chaperone proteins are responsible for hydrolyzing ATP to ADP, harnessing the energy, and then taking folded proteins and unfolding them.[9] Next, Clp-ATPases slip the unfolded proteins into the degradation chamber within ClpP, allowing for processive degradation of the substrate.[8][10] This process is tightly regulated with the hydrolysis of ATP to prevent uncontrolled protein or peptide degradation that would be harmful to the cell.[4]
In contrast, ADEP activates ClpP without the need for ATP hydrolysis, causing degradation of unfolded proteins and peptides within the cell at uncontrolled rates.[8] ADEPs are thought to bind slightly cooperatively on the surface of each ClpP ring in its hydrophobic pockets and have allosteric effects in activation of ClpP.[8] This binding initiates ClpP to undergo a conformational change such that its N-terminal region opens up its axial pore to allow for partial degradation of products, as compared to processive degradation with ClpA.[8] ADEP activation of ClpP does not allow for folded protein degradation, but even with unfolded protein and peptide degradation, ADEP still causes bacterial cell death.[8]
Research has shown that ADEP-activated ClpP targets cell division rather than metabolic processes.[6] ADEP appears to initiate ClpP to preferably degrade FtsZ, an important bacterial protein involved in septum formation that is necessary for bacterial cell division.[6] As a result, Gram-positive bacteria treated with ADEPs form long filaments before cell death.[4][6]
Molecular modification
In order to develop a useful antibiotic, ADEP continues to be modified for greater antimicrobial activity and stability. By restricting components of ADEP to decrease the molecule's flexibility, binding was enhanced and antimicrobial activity significantly increased.[3] Specific amino acids essential to the peptidolactone core of ADEP were altered and restricted, causing stabilization of ADEP in a bioactive conformation.[3] In fact, the conformational restrictions of ADEP resulted in its ability to activate ClpP increasing seven-fold and its antimicrobial activity 1200-fold.[3] Research on altering ADEP molecules continues in attempt to construct a new antibiotic for public use.
References
- ↑ K. H. Michel, R. E. Kastner (Eli Lilly and Company), US 4492650, 1985 [Chem. Abstr. 1985, 102, 130459].
- ↑ Osada, Hiroyuki, Tatsuya Yano, Hiroyuki Koshino, and Kiyoshi Isono. "Enopeptin A, a novel depsipeptide antibiotic with anti-bacteriophage activity." The Journal of Antibiotics 44.12 (1991): 1463-1466. Print.
- 1 2 3 4 5 6 Carney, Daniel W., Karl R. Schmitz, Jonathan V. Truong, Robert T. Sauer, and Jason K. Sello. "Restriction of the Conformational Dynamics of the Cyclic Acyldepsipeptide Antibiotics Improves Their Antibacterial Activity." JACS 136 (2014): 1922-1929. Print.
- 1 2 3 4 5 6 7 Hinzen, Berthold, Harald Labischinski, Heike Brötz-Oesterhelt, Rainer Endermann, Jordi Benet-Buchholz, Veronica Hellwig, Dieter Häbich, Andreas Schumacher, Thomas Lampe, Holger Paulsen, and Siegfried Raddatz. "Medicinal Chemistry Optimization of Acyldepsipeptides of the Enopeptin Class Antibiotics." ChemMedChem 1.7 (2006): 689-693. Print.
- ↑ Brötz-Oesterhelt, Heike, Hans-Georg Sahl, Hein-Peter Kroll, Dieter Beyer, Harald Labischinski, Julia E Bandow, Kerstin Henninger, Holger Paulsen, Siegfried Raddatz, Berthold Hinzen, Werner Schroeder, Christoph Ladel, and Rainer Endermann. "Dysregulation Of Bacterial Proteolytic Machinery By A New Class Of Antibiotics." Nature Medicine 11.10 (2005): 1082-1087. Print.
- 1 2 3 4 Sass, Peter, Michaele Josten, Kirsten Famulla, Guido Schiffer, Hans-Georg Sahi, Leendert Hamoen, and Heike Brotz-Oesterhelt. "Antibiotic acyldepsipeptides activate ClpP peptidase to degrade the cell division protein FtsZ." PNAS 108.42 (2011): 17474-17479. Print.
- ↑ Gominet, M., N. Seghezzi, and P. Mazodier. "Acyl depsipeptide (ADEP) resistance in Streptomyces." Microbiology 157.8 (2011): 2226-2234. Print.
- 1 2 3 4 5 6 7 Li, Dominic Him Shun, Alba Guarné, Michael R. Maurizi, Yi-Qiang Cheng, Gerard D. Wright, Rodolfo Ghirlando, Ebenezer Joseph, Melanie Gloyd, Yu Seon Chung, and Joaquin Ortega. "Acyldepsipeptide Antibiotics Induce The Formation Of A Structured Axial Channel In ClpP: A Model For The ClpX/ClpA-Bound State Of ClpP." Chemistry & Biology 17.9 (2010): 959-969. Print.
- ↑ Hoskins, J. R.. "The role of the ClpA chaperone in proteolysis by ClpAP." Proceedings of the National Academy of Sciences 95.21 (1998): 12135-12140. Print.
- ↑ Ishikawa, T., Beuron, F., Kessel, M., Wickner, S., Maurizi, M., and Steven, A. "Translocation pathway of protein substrates in ClpAP protease." Proceedings of the National Academy of Sciences 98.8 (2001): 4328-4333. Print.
Further reading
- Molecular description of ADEP1