3,4-Dihydroxymandelic acid
 | |
| Names | |
|---|---|
| IUPAC name
2-(3,4-dihydroxyphenyl)-2-hydroxyacetic acid | |
| Identifiers | |
| 775-01-9 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:27637 |
| ChemSpider | 77371 |
| ECHA InfoCard | 100.011.154 |
| 6633 | |
| KEGG | C05580 |
| MeSH | 3,4-dihydroxymandelic+acid |
| PubChem | 85782 |
| |
| |
| Properties | |
| C8H8O5 | |
| Molar mass | 184.14612 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
3,4-Dihydroxymandelic acid (DHMA, DOMA) is a metabolite of norepinephrine.[1]
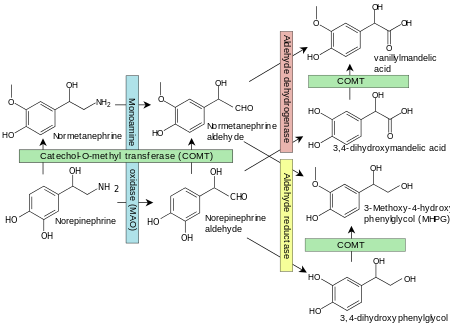
Norepinephrine degradation. 3,4-Dihydroxymandelic acid is shown at right. Enzymes are shown in boxes.[2]
References
- ↑ Ley JP; Engelhart K; Bernhardt J; Bertram HJ (October 2002). "3,4-Dihydroxymandelic acid, a noradrenalin metabolite with powerful antioxidative potential". J. Agric. Food Chem. 50 (21): 5897–902. doi:10.1021/jf025667e. PMID 12358456.
- ↑ Figure 11-4 in: Rod Flower; Humphrey P. Rang; Maureen M. Dale; Ritter, James M. (2007). Rang & Dale's pharmacology. Edinburgh: Churchill Livingstone. ISBN 0-443-06911-5.
This article is issued from Wikipedia - version of the 5/17/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.