Type VI secretion system
The type VI secretion system (T6SS) is a one step mechanism that is used widely throughout gram-negative bacterial species in injecting effector proteins and virulence factors (such as proteins, toxins, or enzymes) from across the interior (cytoplasm or cytosol) of a bacterial cell into a target cell. T6SS were first identified in 2006, when researchers at Harvard Medical School (Boston, United States) saw that mutations of Hcp and VgrG proteins in Vibrio cholerae led to decreased virulence and infectivity of the pathogen.[1] Soon afterwards, a T6SS was found in Pseudomonas aeruginosa and implicated with Hcp secretion into target cells and chronic infection.[2]
Since then, Type VI secretion systems have been found in a quarter of all proteobacterial genomes, including animal, plant, and human pathogens, as well as soil, environmental or marine bacteria.[3] While most of the early studies of Type VI secretion focused on its role in the pathogenesis of higher organisms, more recent studies suggest a broader physiological role in defense against simple eukaryotic predators and its role in inter-bacterial interactions.
Architecture
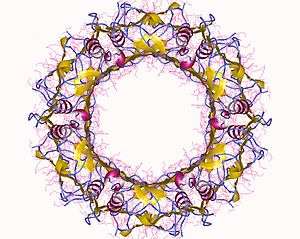
The Type VI Secretion system is a complex structure composed of 13 conserved proteins (which form the core subunit) and a variable amount of accessory proteins. It is often referred to as an inverted phage structure, with a 'puncturing' device at the tip of the complex which can cross target cell membranes to inject effector proteins.

Core and Accessory Components
Hcp
Among the most conserved proteins in different type VI secretion systems is Hcp (haemolysin-coregulated protein), which carries a resemblance to various bacteriophage tail tube proteins,[4] and is in fact a structural homolog of lambda phage tube protein p5. Hcp crystal structures show stacked rings of Hcp hexamers forming a tubular structure [5] through which proteins passage to be delivered to the target cell.
VgrG
VgrG (valine-glycine repeat G) is a T6SS protein that forms a trimer that shows significant structural homology to the needle protein complex of the T4 Phage (gp27 and gp5 proteins). VrgG is displayed at the tip of the Hcp tubule and is propelled towards the target cell.[6] The tip of VgrG is too narrow to allow protein passage into target cells and so it is believed that after piercing through the target cell, VgrG dissociates and allows Hcp to be exposed to the target.[6]
Some VrgG proteins have evolved to contain additional C-terminal domains with effector functions, usually enzymatic. A hallmark example for this in Vibrio cholerae, where the extended VgrG domain VgrG-1 has actin cross linking activity. This allows Vibrio in phagocytic vacuoles, to puncture the vacuole membrane, releasing VgrG-1 into the phagocytic cytosol. Actin crosslinking domain is translocated into the phagocytic cytosol, where it can cause G-actin crosslinking. This results in the inhibition of F-actin formation, so the phagocyte can no longer phagocytose any more Vibrio Cholerae. This altruistic mechanism is a clever method by which Vibrio can escape innate immune responses.[7]
VipA and VipB
VipA and VipB (in Vibrio Cholerae) are proteins which form tubule-like structures at the base of the T6SS machinery in an ATPase-dependent manner. In 2009, it was noted that these proteins were very similar to contracted T4 phage tails.[4] Crystal structure studies show that the VipA/VipB tubule is large enough to accommodate the Hcp tubule, which suggests that the VipA/VibB tubule functions as a contractile sheath, pushing the Hcp tube from the inside of the bacterial membrane out.
ATPase
Like other secretion systems, the T6SS has an ATPase which acts as a motor in effector protein translocation into target cells. T6SS ATPases resemble AAA+ ATPase proteins.
In Vibrio Cholerae, the ATPase ClpV was among the first T6SS proteins studied. ClpV forms a hexameric ring at the base of the T6SS apparatus. ClpV mutants have static VipA/B tubules and it has also been shown that ClpV binds to the contracted conformation of these tubules. It has been proposed that ClpV is not responsible for tubule assembly, but rather for depolymerisation (remodelling/recycling) of VipA and VipB tubules allowing their contraction.[8]
Baseplate Complex
T6SS apparatus requires attachment to the bacterial membrane in order to be able to translocate effectors into target cells. In bacteriophages, a baseplate platform manages this task, but in T6SS, a complex related to Legionella proteins DotU and IcmF has been found responsible for attachment.[9][10] DotU (TssL) is thought to anchor the complex to the peptidoglycan layer through an extended domain, or through another additional accessory protein.[11] Together, TssL, TssM (IcmF) and TssJ (an additional outer membrane lipoprotein) form the “baseplate complex”, however it is unknown how this is anchored to the tube and sheath proteins. Studies suggest that TssK, which is a universally conserved protein in most T6SS, might be the anchor between the TssL-TssM-TssJ complex and VipA/VipB tubules, as well as with Hcp.
Other Components
TssF, TssA, TssG are three other proteins with a high degree of conservation across T6SS, however their structure and specific function remains to be yet discovered.[12]
Anti-Eukaryotic Type VI Secretion Systems
Type VI secretion systems can inject effectors into eukaryotic cells that interrupt proper cell function. Among these are the ‘evolved’ VgrG effectors, notably the VgrG-1 protein of Vibrio cholerae, which has an actin crosslinking domain,[7] or VgrG2b from ''P. aeruginosa'' which targets microtubules, and more particularly the gamma-tubulin ring complex.[13] Other examples include VgrG-1 in Aeromonas hydrophila, which has an ADP-ribosylating domain which can be injected into phagocytic cytosol, where it inhibits actin polymerization by increasing the G:F actin ratio. Some of the VgrG domain extensions are also thought to act as delivery vehicles for bacterial effector protein – for example, TseL (type six effector lipase) in Vibrio cholerae interacts with VgrG-3 for its delivery into target cells.[14] T.G. Sana; C. Baumann; A. Merdes; C. Soscia; T. Rattei; A. Hachani; C. Jones; K.L. Bennett; A. Filloux; G. Superti-Furga; R. Voulhoux; S. Bleves (2015). "Internalization of Pseudomonas aeruginosa Strain PAO1 into Epithelial Cells Is Promoted by Interaction of a T6SS Effector with the Microtubule Network". MBio. 2;6: (3):e00712. doi:10.1128/mBio.00712-15.,</ref name="Sana 2015">
Antibacterial Type VI Secretion Systems

Type VI secretion system effectors have been identified which instead of acting against eukaryotic cells, act against other bacterial species. Effectors such as Tse1, Tse2 and Tse3 in P. aeruginosa can cause peptidoglycan cell wall lysis (Tse 1 and Tse 3) as well as cell senescence by an unknown mechanism (Tse2).[15] As a defense mechanism against Tse toxins, P. aeruginosa produces Tsi proteins (1-3), which can bind and neutralize internal Tse proteins, preventing intraspecies antagonization. This is a novel mechanism by which T6SS gives bacteria the ability to compete other species without harming their own self. Such a mechanism has also been found in species such as S. marcescens, where two related T6SS toxins also have self-resistant neutralizers.
Regulation of Type VI Secretion
GacS/Rsm Two Component System
Some research has gone into regulation of T6SS by two component systems. In P. aeruginosa, it has been observed that the GacS/Rsm two-component system is involved in type VI secretion system regulation. This system regulates the expression of Rsm small regulatory RNA molecules, and has also been implicated in biofilm formation. Upon the GacS/Rsm pathway stimulation, an increase in Rsm molecules leads to inhibition of mRNA-binding protein RsmA. RsmA is a translational inhibitor that binds to sequences near the ribosome-binding site for T6SS gene expression. This level of regulation has also been observed in P. fluorescens and P. syringae [5][16]
Quorum Sensing
There are various examples in which quorum sensing regulates T6SS. In Vibrio cholerae T6SS studies, it has been observed that serotype O37 has high vas gene expression. Serotypes O139 and O1 on the other hand exhibit the opposite, with markedly low vas gene expression. It has been suggested that the differences in expression are attributable to differences in quorum-sensing levels. In Vibrio cholerae, autoinducer-1 (AI-1) signals are detected by LuxQ, a sensor kinase. LuxQ activates LuxU, which then acts on LuxO, a DNA-binding protein which represses HapR gene expression. HapR is thought to LuxO deletions resulted in strong induction of vas gene expression, and hence T6SS expression, demonstrating that T6SS is regulated in some form by quorum sensing.[17] However, O1 strains with LuxO deletions still had relatively quiescent T6SS compared to the O37 strain, suggesting that additional factors are also involved[6] .
See also
References
- ↑ Pukatzki, Stefan; Ma, Amy T.; Sturtevant, Derek; Krastins, Bryan; Sarracino, David; Nelson, William C.; Heidelberg, John F.; Mekalanos, John J. (2006). "'Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system'". Proceedings of the National Academy of Sciences. 103: 1528–33. doi:10.1073/pnas.0510322103. PMC 1345711
 . PMID 16432199.
. PMID 16432199.
- ↑ Mougous, Joseph D.; Cuff, Marianne E.; Raunser, Stefan; Shen, Aimee; Zhou, Min; Gifford, Casey A.; Goodman, Andrew L.; Joachimiak, Grazyna; et al. (2006). "A Virulence Locus of Pseudomonas aeruginosa Encodes a Protein Secretion Apparatus". Science. 312 (5779): 1526–30. Bibcode:2006Sci...312.1526M. doi:10.1126/science.1128393. PMC 2800167
 . PMID 16763151.
. PMID 16763151. - ↑ Bingle, Lewis EH; Bailey, Christopher M; Pallen, Mark J (2008). "Type VI secretion: A beginner's guide". Current Opinion in Microbiology. 11 (1): 3–8. doi:10.1016/j.mib.2008.01.006. PMID 18289922.
- 1 2 Petr G. Leimana, Marek Baslerb, Udupi A. Ramagopalc, Jeffrey B. Bonannoc, J. Michael Sauderd, Stefan Pukatzkie, Stephen K. Burleyd, Steven C. Almoc and John J. Mekalanosbz (2009). "Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin". Proceedings of the National Academy of Sciences. 106: 4154–4159. doi:10.1073/pnas.0813360106. PMC 2657435
 . PMID 19251641.
. PMID 19251641.
- 1 2 Joseph D. Mougous; Marianne E. Cuff; Stefan Raunser; Aimee Shen; Min Zhou; Casey A. Gifford; Andrew L. Goodman; Grazyna Joachimiak; Claudia L. Ordoñez; Stephen Lory; Thomas Walz; Andrzej Joachimiak; John J. Mekalanos (2006). "A Virulence Locus of Pseudomonas aeruginosa Encodes a Protein Secretion Apparatus". Science. 312: 1526–1530. doi:10.1126/science.1128393. PMC 2800167
 . PMID 16763151.
. PMID 16763151. - 1 2 3 Julie M. Silverman; Yannick R. Brunet; Eric Cascales; Joseph D. Mougous (2012). "Structure and Regulation of the Type VI Secretion System". Annual Review of Microbiology. 66: 453–472. doi:10.1146/annurev-micro-121809-151619. PMC 3595004
 . PMID 22746332.
. PMID 22746332.
- 1 2 A.T. Ma; S. McAuley; S. Pukatzki; J.J. Mekalanos (2009). "Translocation of a Vibrio cholerae type VI secretion effector requires bacterial endocytosis by host cells". Cell Host Microbe. 5: 234–243. doi:10.1016/j.chom.2009.02.005.
- ↑ Bönemann, G., Pietrosiuk, A., Diemand, A., Zentgraf, H., and Mogk, A. (2009). "Remodelling of VipA/VipB tubules by ClpV-mediated threading is crucial for type VI protein secretion". EMBO. 28: 315–325. doi:10.1038/emboj.2008.269.
- ↑ E. Durand; A. Zoued; S. Spinelli; P.J. Watson; M.S. Aschtgen; L. Journet; C. Cambillau; E. Cascales. (2012). "Structural characterization and oligomerization of the TssL protein, a component shared by bacterial type VI and type IVb secretion systems". J. Biol. Chem. 287: 14157–14168. doi:10.1074/jbc.M111.338731.
- ↑ Lay-Sun Ma; Jer-Sheng Lin; Erh-Min Lai (2009). "An IcmF Family Protein, ImpLM, Is an Integral Inner Membrane Protein Interacting with ImpKL, and Its Walker A Motif Is Required for Type VI Secretion System-Mediated Hcp Secretion in Agrobacterium tumefaciens". J. Bacteriol. 191: 4316–4329. doi:10.1128/JB.00029-09.
- ↑ Aschtgen, Marie-Stéphanie S., Mark, Cascales, Eric (2010). "Anchoring the type VI secretion system to the peptidoglycan: TssL, TagL, TagP... what else?". Virulence. 1: 535–540. doi:10.4161/viru.1.6.13732.
- ↑ Dong Tao; Ho Brian T.; Mekalanos John (2014). "A View to a Kill: The Bacterial Type VI Secretion System". Cell Host and Microbe. 15: 9–21.
- ↑
- ↑ T.G. Dong; B.T. Ho; D.R. Yoder-Himes; J.J. Mekalanos (2013). "Identification of T6SS-dependent effector and immunity proteins by Tn-seq in Vibrio cholerae". Cell Host Microbe. 110: 2623–2628. doi:10.1073/pnas.1222783110.,
- ↑ Hood, R. D., Singh, P., Hsu, F., Güvener, T., Carl, M. A., Trinidad, R. R. S., Silverman, J. M., Ohlson, B. B., Hicks, K. G., Plemel, R. L., Li, M., Schwarz, S., Wang, W. Y., Merz, A. J., Goodlett, D. R., and Mougous, J. D. (2010). "A Type VI secretion system of Pseudomonas Aeruginosa targets a toxin to bacteria". Cell Host Microbe. 7: 25–37. doi:10.1073/pnas.1222783110.
- ↑ Records AR, Gross DC (2010). "Sensor kinases RetS and LadS regulate Pseudomonas syringae type VI secretion and virulence factors". J. Bacteriol. 192: 3584–96. doi:10.1128/JB.00114-10.
- ↑ Ishikawa T, Rompikuntal PK, Lindmark B, Milton DL, Wai SN (2009). "Quorum sensing regulation of the two hcp alleles in Vibrio cholerae O1 strains". PLoS One. 4: 3584–3596. doi:10.1128/JB.00114-10.