Trimethylphosphine
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Trimethylphosphane | |
| Systematic IUPAC name | |
| Identifiers | |
| 594-09-2 | |
| 3D model (Jmol) | Interactive image |
| 969138 | |
| ChEBI | CHEBI:35890 |
| ChemSpider | 62205 |
| ECHA InfoCard | 100.008.932 |
| EC Number | 209-823-1 |
| MeSH | trimethyl+phosphine |
| PubChem | 68983 |
| UN number | 1993 |
| |
| |
| Properties | |
| C3H9P | |
| Molar mass | 76.08 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 735 mg cm−3 |
| Melting point | −86 °C (−123 °F; 187 K) |
| Boiling point | 38 to 39 °C (100 to 102 °F; 311 to 312 K) |
| Vapor pressure | 49.9 kPa (at 20 °C) |
| Structure | |
| Trigonal pyramidal | |
| 1.19 Debye | |
| Hazards | |
| GHS pictograms |   |
| GHS signal word | DANGER |
| H225, H315, H319, H335 | |
| P210, P261, P305+351+338 | |
| EU classification (DSD) |
|
| R-phrases | R11, R36/37/38 |
| S-phrases | S9, S16, S26, S36/37/39 |
| Flash point | −19 °C (−2 °F; 254 K) |
| Related compounds | |
| Related compounds |
NMe3 PH3 PPh3 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Trimethylphosphine is the organophosphorus compound with the formula P(CH3)3, commonly abbreviated as PMe3. This colorless liquid has a strongly unpleasant odor, which is characteristic of alkylphosphines. The compound is a common ligand in coordination chemistry.
Structure and bonding
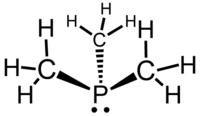
It is a pyramidal molecule with approximate C3v symmetry — the molecular point group of ammonia (NH3) and phosphine (PH3). The C–P–C bond angles are approximately 98.6°.[2]
The C–P–C bond angles are consistent with the notion that phosphorus predominantly uses the 3p orbitals for forming bonds and that there is little sp hybridization of the phosphorus atom. The latter is a common feature of the chemistry of phosphorus because the gap between the 3s and 3p orbital is large compared to the corresponding gap for the 2s and 2p orbitals that are in the valence shell for carbon, nitrogen, and oxygen (elements for which sp-mixing is strong). As a result, the lone pair of trimethylphosphine has predominantly s-character as is the case for phosphine, PH3.[3]
PMe3 is usually be prepared by the treatment of triphenyl phosphite with methylmagnesium chloride:[4]
- 3 CH3MgCl + P(OC6H5)3 → P(CH3)3 + 3 C6H5OMgCl
The synthesis is conducted in dibutyl ether, from which the more volatile PMe3 can be distilled.
Reactions
Acid-base reactions
With a pKa of 8.65, PMe3 reacts with strong acids to give salts [HPMe3]X.[2] This reaction is reversible. WIth strong bases, such as alkyl lithium compounds, a methyl group undergoes deprotonation to give PMe2CH2Li.
PMe3 is easily oxidised to the phosphine oxide with oxygen. It is also easily alkylated to give phosphonium derivatives RPMe3+. The compound is stable to water.
Coordination chemistry
Trimethylphosphine is a highly basic ligand that forms complexes with most metals. As a ligand, trimethylphosphine's Tolman cone angle is 118°.[5] This angle is an indication of the amount of steric protection that this ligand provides to the metal that to which it is bound.
Being a relatively compact ligand, several can bind to a single transition metal, as illustrated by this Fe(0) complex:
- 2 PMe3 + Fe(CO)5 → Fe(CO)3(PMe3)2 + 2 CO
Its complex with silver iodide, AgI(PMe3) is an air-stable solid that releases PMe3 upon heating.
Safety
PMe3 is potentially pyrophoric as well as toxic. PMe3 converts to non-pyrophoric phosphine oxide by treatment with dilute bleach.
References
- 1 2 "Trimethylphosphine (CHEBI:35890)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute. 6 June 2006. IUPAC Names. Retrieved 25 September 2011.
- 1 2 Annette Schier and Hubert Schmidbaur "P-Donor Ligands" in Encyclopedia of Inorganic Chemistry 2006, Wiley-VCH, Weinheim. doi:10.1002/0470862106.ia177
- ↑ E. Fluck, The chemistry of phosphine, Topics in Current Chemistry Vol. 35, 64 pp, 1973.
- ↑ Leutkens, Jr., M. L.; Sattelberger, A. P.; Murray, H. H.; Basil, J. D.; Fackler, Jr. J. P. (1990). Robert J. Angelici, ed. "Trimethylphosphine". Inorganic Syntheses. Inorganic Syntheses. New York: J. Wiley & Sons. 28: 305–310. doi:10.1002/9780470132593.ch76. ISBN 0-471-52619-3.
- ↑ G. L. Miessler and D. A. Tarr Inorganic Chemistry, 3rd Ed, Pearson/Prentice Hall publisher, ISBN 0-13-035471-6.