Thiocarbamoyl chloride
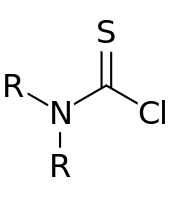
General chemical structure of a thiocarbamoyl chloride
A thiocarbamoyl chloride is an organosulfur compound with the formula R2NC(S)Cl (R = alkyl, aryl, etc.).[1] These compounds are electrophiles, serving as a source of R2NC(S)+. They are analogous to carbamoyl chlorides, which have the formula R2NC(O)Cl. A common example of this class of compound is dimethylthiocarbamoyl chloride, Me2NC(S)Cl (CAS#16420-13-6), which is a pale yellow, volatile solid.
Synthesis and reactions
Thiocarbamoyl chlorides are prepared by chlorination of the related thiuram disulfides:
- [R2NC(S)]2S2 + 3 Cl2 → 2 R2NC(S)Cl + 2 SCl2
Thiocarbamoyl chlorides react with dithiocarbamates (R2NCS−
2) to give thiuram sulfides [R2NC(S)]2S.
References
- ↑ R. J. Cremlyn “An Introduction to Organosulfur Chemistry” John Wiley and Sons: Chichester (1996). ISBN 0 471 95512 4.
This article is issued from Wikipedia - version of the 10/9/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.