Selexipag
 | |
| Names | |
|---|---|
| IUPAC name
2-{4-[(5,6-diphenylpyrazin-2-yl)(propan-2-yl)amino]butoxy}-N-(methanesulfonyl)acetamide | |
| Other names
ACT-293987, NS-304 | |
| Identifiers | |
| 475086-01-2 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:90844 |
| ChEMBL | ChEMBL238804 |
| ChemSpider | 8089417 |
| ECHA InfoCard | 100.237.916 |
| 7552 | |
| KEGG | D09994 |
| PubChem | 9913767 |
| UNII | P7T269PR6S |
| |
| |
| Properties | |
| C26H32N4O4S | |
| Molar mass | 496.6 g·mol−1 |
| Pharmacology | |
| B01AC27 (WHO) | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Selexipag (brand name Uptravi) is a drug developed by Actelion for the treatment of pulmonary arterial hypertension (PAH). Selexipag and its active metabolite, ACT-333679 (or MRE-269, the free carboxylic acid), are agonists of the prostacyclin receptor, which leads to vasodilation in the pulmonary circulation.[1]
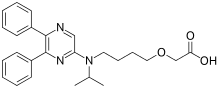
ACT-333679 or MRE-269, the active metabolite of selexipag
History
The US FDA granted selexipag Orphan Drug status for PAH.[2] It was approved by the US FDA on 22 December 2015.[2] The expected price for the drug in the US is $160,000 to $170,000 per patient before rebates.[3]
See also
- Epoprostenol, another name for prostacyclin
- Analogues of prostacyclin:
References
- ↑ Sitbon, O.; Morrell, N. (2012). "Pathways in pulmonary arterial hypertension: The future is here". European Respiratory Review. 21 (126): 321–327. doi:10.1183/09059180.00004812. PMID 23204120.
- 1 2 New Drug Approved for Rare Lung Disorder. PPN. 23 Dec 2015 Has link to GRIPHON study results
- ↑ "Actelion sees Uptravi price of $160,000-170,000/patient". Reuters. 2016-01-05. Retrieved 2016-01-06.
This article is issued from Wikipedia - version of the 1/7/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.