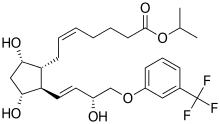
Travoprost
 | |
| Clinical data | |
|---|---|
| Trade names | Travatan |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a602027 |
| Pregnancy category |
|
| Routes of administration | Topical (eye drops) |
| ATC code | S01EE04 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Activation by ester hydrolysis, deactivation by beta oxidation, OH-oxidation, double bond reduction |
| Onset of action | 2 hours |
| Biological half-life |
1.5 hours (in aqueous fluid) 45 minutes (terminal) |
| Duration of action | ≥ 24 hours |
| Excretion | Mainly via kidney |
| Identifiers | |
| |
| CAS Number |
157283-68-6 |
| PubChem (CID) | 5282226 |
| IUPHAR/BPS | 7102 |
| DrugBank |
DB00287 |
| ChemSpider |
4445407 |
| UNII |
WJ68R08KX9 |
| KEGG |
D01964 |
| ChEBI |
CHEBI:746859 |
| ChEMBL |
CHEMBL1200799 |
| ECHA InfoCard | 100.207.141 |
| Chemical and physical data | |
| Formula | C26H35F3O6 |
| Molar mass | 500.548 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Travoprost ophthalmic solution is a topical medication used for controlling the progression of glaucoma or ocular hypertension, by reducing intraocular pressure. It is a synthetic prostaglandin analog (or more specifically, an analog of prostaglandin F2α)[1][2] that works by increasing the outflow of aqueous fluid from the eyes.[3] It is also known by the brand names of Travatan and Travatan Z, manufactured by Alcon.
Side effects
Possible side effects are:[4]
- blurred vision
- eyelid redness
- permanent darkening of eyelashes
- eye discomfort
- permanent darkening of the iris to brown (heterochromia)
- burning sensation during use
- thickening of the eyelashes
- inflammation of the prostate gland, restricting urine flow (BPH)
Pharmacology
Mechanism of action
Like other analogs of prostaglandin F2α such as tafluprost and latanoprost, travoprost is an ester prodrug of the free acid, which acts as an agonist at the prostaglandin F receptor, increasing outflow of aqueous fluid from the eye and thus lowering intraocular pressure.[4]
Pharmacokinetics
Travoprost is absorbed through the cornea, where it is hydrolysed to the free travoprost acid. Highest concentrations of the acid in the eye are reached one to two hours after application, and its half-life in the aqueous fluid is 1.5 hours. Once it reaches the bloodstream, it is quickly metabolised, so that concentrations in the system do not exceed 25 pg/ml (compared to 20 ng/ml in the eye, which is higher by nearly a factor of 1000).[4]
Metabolites are formed by beta oxidation of the acidic chain (compare Tafluprost#Pharmacokinetics), oxidation of the OH-group in the other side chain, and reduction of the double bond next to this OH-group. Travoprost acid and its metabolites are mainly excreted via the kidney[4] with a terminal half-life of 45 minutes.[5]

References
- ↑ Alcon Laboratories, Inc. (September 2011). "TRAVATAN - travoprost solution". DailyMed. Bethesda, MD: U.S. National Library of Medicine. Retrieved 2011-09-30.
- ↑ Alcon Laboratories, Inc. (September 2011). "TRAVATAN Z (travoprost) solution". DailyMed. Bethesda, MD: U.S. National Library of Medicine. Retrieved 2011-09-30.
- ↑ AHFS Consumer Medication Information (2011-01-01). "Travoprost Ophthalmic". MedlinePlus. Bethesda, MD: U.S. National Library of Medicine. Retrieved 2011-09-30.
- 1 2 3 4 Haberfeld, H, ed. (2015). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag.
- ↑ Drugs.com: Travoprost Monograph.