Rhodanine
Not to be confused with rhodamine.
 | |
| Names | |
|---|---|
| IUPAC name
2-Sulfanylidene-1,3-thiazolidin-4-one | |
| Other names
2-Thioxo-4-thiazolidinone; 4-Oxo-2-thioxothiazoline | |
| Identifiers | |
| 141-84-4 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL224633 |
| ChemSpider | 1013337 |
| ECHA InfoCard | 100.005.005 |
| PubChem | 1201546 |
| |
| |
| Properties | |
| C3H3NOS2 | |
| Molar mass | 133.18 g·mol−1 |
| Melting point | 165 to 169 °C (329 to 336 °F; 438 to 442 K) |
| Hazards | |
| EU classification (DSD) |
|
| R-phrases | R22 R41 |
| S-phrases | S22 S26 S39 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Rhodanine is a 5-membered heterocyclic organic compound possessing a thiazolidine core. It was first discovered in 1877 by Marceli Nencki who named it "Rhodaninsaure" in reference to its synthesis from ammonium rhodanide (in modern chemistry ammonium thiocyanate) and chloroacetic acid in water.[2]
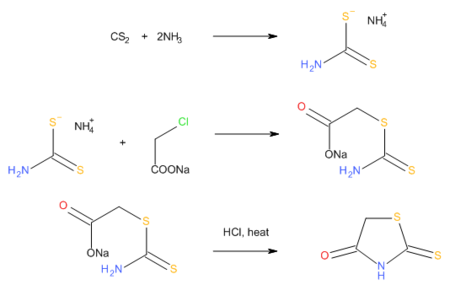
Rhodanines can also be prepared by the reaction of carbon disulfide, ammonia, and chloroacetic acid, which proceeds via an intermediate dithiocarbamate.[3]
Some rhodanine derivatives have pharmacological properties; for instance, epalrestat is used to treat diabetic neuropathy. However, most are promiscuous binders with poor selectivity; as a result, this class of compounds is viewed with suspicion by medicinal chemists,[4][5][6]
References
- ↑ Rhodanine at Sigma-Aldrich
- ↑ Nencki, M. (10 July 1877). "Ueber die Einwirkung der Monochloressigsäure auf Sulfocyansäure und ihre Salze". Journal fur Praktische Chemie. 16 (1): 1–17. doi:10.1002/prac.18770160101.
- ↑ C. Ernst Redemann; Roland N. Icke; Gordon A. Alles (1955). "Rhodanine". Org. Synth.; Coll. Vol., 3, p. 763
- ↑ J.B. Baell, G.A. Holloway (2010). "New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays". J. Med. Chem. 53 (7): 2719–2740. doi:10.1021/jm901137j. PMID 20131845.
- ↑ Tomašić, Tihomir; Peterlin Mašič, Lucija (2012). "Rhodanine as a scaffold in drug discovery: A critical review of its biological activities and mechanisms of target modulation". Expert Opinion on Drug Discovery: 1. doi:10.1517/17460441.2012.688743.
- ↑ Pouliot, Martin; Jeanmart, Stephane (8 September 2015). "Pan Assay Interference Compounds (PAINS) and Other Promiscuous Compounds in Antifungal Research". Journal of Medicinal Chemistry: 150908163303002. doi:10.1021/acs.jmedchem.5b00361.
This article is issued from Wikipedia - version of the 11/28/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.