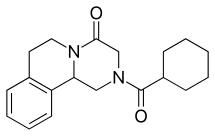
Praziquantel
 | |
| Clinical data | |
|---|---|
| Pronunciation | praz-i-KWAN-tel |
| Trade names | Biltricide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608048 |
| Pregnancy category | |
| Routes of administration | Human use: by mouth (tablets) |
| ATC code | P02BA01 (WHO) QP52AA01 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Relatively small |
| Metabolism | Liver |
| Biological half-life | 0.8–1.5 hours (main metabolites: 4–5 hours) |
| Excretion | Urine (mainly) |
| Identifiers | |
| |
| CAS Number |
55268-74-1 |
| PubChem (CID) | 4891 |
| DrugBank |
DB01058 |
| ChemSpider |
4722 |
| UNII |
6490C9U457 |
| KEGG |
D00471 |
| ChEMBL |
CHEMBL976 |
| Chemical and physical data | |
| Formula | C19H24N2O2 |
| Molar mass | 312.411 |
| 3D model (Jmol) | Interactive image |
| Melting point | 136 to 138 °C (277 to 280 °F) |
| |
| |
| (verify) | |
Praziquantel, marketed as Biltricide, is an anthelmintic used for the treatment of tapeworms and flukes. For example, it is effective against Schistosoma (blood flukes), the liver fluke Clonorchis sinensis and the fish tapeworm Diphyllobothrium latum.
It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[2]
Medical uses
Praziquantel is used to treat diseases caused by infection with several types of internal/gastrointestinal, and external parasites, including:
- Hydatid disease caused by infection of various organs with larval stages of tapeworms of the genus Echinococcus.
- Cysticercosis caused by infection of the brain and/or muscles with the eggs and larvae of the pork tapeworm Taenia solium (though it has been judged less effective than albendazole in treatment of neurocysticercosis).[3]
- In cats and dogs whose gastrointestinal tract is infected with the tapeworms Dipylidium caninum or Taenia taeniaeformis, respectively;[4][5] praziquantel is also often used in fixed combination with pyrantel embonate against the roundworms (ascarids): Toxocara cati and Toxascaris leonina.[4][5] Praziquantel is also effective against Echinococcus multilocularis.[4]
- Schistosomiasis caused by trematodes of the genus Schistosoma.[6] As of 2005, praziquantel is the primary treatment for human schistosomiasis, for which it is usually effective in a single dose.[7]
- Clonorchiasis brought on by the Chinese liver fluke Clonorchis sinensis.[8]
- Paragonimiasis caused by infection with lung flukes, mostly of the species Paragonimus westermani.
- Fasciolopsiasis caused by intestinal fluke Fasciolopsis buski.[9]
Side effects
The majority of side effects develop due to the release of the contents of the parasites as they are killed and the consequent host immune reaction. The heavier the parasite burden, the heavier and more frequent the side effects normally are.
- Central nervous system: Frequently occurring side effects are dizziness, headache, and malaise. Drowsiness, somnolence, fatigue, and vertigo have also been seen. Almost all patients with cerebral cysticercosis experience CNS side effects related to the cell-death of the parasites (headache, worsening of pre-existing neurological problems, seizures, arachnoiditis, and meningism). These side effects may be life-threatening and can be reduced by coadministration of corticosteroids. It is strongly recommended that all patients with cerebral cysticercosis are hospitalized during treatment.
- Gastrointestinal tract: Approximately 90% of all patients have abdominal pain or cramps with or without nausea and vomiting. Diarrhea may develop and may be severe with colic. Sweating, fever, and sometimes bloody stools may occur together with diarrhea.
- Liver: Asymptomatic and transient increases of liver enzymes (AST and ALT) are noted frequently (up to 27%). No case of symptomatic liver damage has ever been seen so far.
- Sensitivity reactions: Urticaria, rash, pruritus and eosinophilia in white blood cell counts
- Other locations/body as a whole: Lower back pain, myalgia, arthralgia, fever, sweating, various cardiac arrhythmias, and hypotension
Pregnancy
Animal studies have failed to reveal evidence of fetal harm. There are no controlled data in human pregnancy. Praziquantel should be used during pregnancy only if clearly needed; caution is recommended.
Drug interactions
The antibiotic rifampicin decreases plasma concentrations of praziquantel.[10]
Carbamazepine and phenytoin are reported to reduce the bioavailability of praziquantel.[11]
Chloroquine reduces the bioavailability of praziquantel.[12]
The drug cimetidine heightens praziquantel bioavailability.[13][14]
Mechanism of action
The mode of action is not exactly known at present, but experimental evidence indicates praziquantel increases the permeability of the membranes of schistosome cells towards calcium ions. The drug thereby induces contraction of the parasites, resulting in paralysis in the contracted state. The dying parasites are dislodged from their site of action in the host organism and may enter systemic circulation or may be destroyed by host immune reaction (phagocytosis). Additional mechanisms including focal disintegrations and disturbances of oviposition (laying of eggs) are seen in other types of sensitive parasites.
Another hypothesis concerning the mechanism of action of praziquantel has been recently reported. The drug seems to interfere with adenosine uptake in cultured worms. This effect may have therapeutical relevance given that the schistosome, as the Taenia and the Echinococcus (other praziquantel-sensitive parasites), is unable to synthesize purines such as adenosine de novo.
Bayer's Animal Health Division website states, "Praziquantel is active against cestodes (tapeworms). Praziquantel is absorbed, metabolized in the liver, and excreted in the bile. Upon entering the digestive tract from the bile, cestocidal activity is exhibited. Following exposure to praziquantel, the tapeworm loses its ability to resist digestion by the mammalian host. Because of this, whole tapeworms, including the scolices (plural of "scolex"), are very rarely passed after administration of praziquantel. In many instances, only disintegrated and partially digested pieces of tapeworms will be seen in the stool. The majority of tapeworms are digested and are not found in the feces."[15]
Praziquantel is administered as a racemate, but only the (R)-enantiomer is biologically active; the enantiomers may be separated using a resolution of an amine obtained from praziquantel.[16]
Pharmacokinetics
Praziquantel is well absorbed (about 80%) from the gastrointestinal tract. However, due to extensive first-pass metabolism, only a relatively small amount enters systemic circulation. Praziquantel has a serum half-life of 0.8 to 1.5 hours in adults with normal renal and liver function. Metabolites have a half-life of 4 to 5 hours. In patients with significantly impaired liver function (Child-Pugh score B and C), the serum half-life is increased to 3 to 8 hours. Praziquantel and its metabolites are mainly excreted renally; within 24 h after a single oral dose, 70 to 80% is found in urine, but less than 0.1% as the unchanged drug. Praziquantel is metabolized through the cytochrome P450 pathway via CYP3A4. Agents that induce or inhibit CYP3A4 such as phenytoin, rifampin, and azole antifungals will affect the metabolism of praziquantel.
Praziquantel has a particularly dramatic effect on patients with schistosomiasis. Studies of those treated have shown that within six months of receiving a dose of praziquantel, up to 90% of the damage done to internal organs due to schistosomiasis infection can be reversed.[7]
History
Praziquantel was developed in the laboratories for parasitological research of Bayer AG and Merck KGaA in Germany (Elberfeld and Darmstadt) in the mid 1970s.
Society and culture
Brand names
- Biltricide (Bayer) Tablets (for human use)[17]
- Cesol (Merck) Tablets
- Cestoved (Vedco) both tablets and injectable for veterinary use
- Cysticide (Merck) Tablets
- Distoside (Chandra Bhagat Pharma Pvt Ltd) tablet (for human use)
- Droncit (Bayer) for veterinary use
- Drontal (combination with pyrantel pamoate) (Bayer) for veterinary use
- D-Worm (Farnum) for veterinary use; note that D-Worm also makes roundworm medicine containing piperidine which is not effective against tapeworms.
- Fish Tapes (Thomas Labs) for aquarium use
- Kaicide (Taiwan)
- Milbemax (combination with milbemycin oxime) (Novartis) for veterinary use
- Popantel (Jurox)
- PraziPro (Hikari) for aquarium use
- Praz-Tastic (NFP/National Fish Pharmaceuticals) for aquarium use
- Pure Prazi (COTS Koi/Children of the Sun Koi) for aquarium use
- PraziPure (J.K.O., Inc. d/b/a Kodama Koi Farm & Kodama Koi Garden; licensed by COTS Koi) for aquarium use
- Profender (combination with emodepside) (Bayer) for veterinary use
- Tape Worm Tabs (Trade Winds) for veterinary use
- Zentozide (Berich (Thailand) Co)
Regulatory approval
Praziquantel is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[2]
Praziquantel is not licensed for use in humans in the UK but it can be imported when necessary on a named patient basis.[18] It is available in the UK as a veterinary anthelmintic.
Praziquantel is FDA approved in the US for the treatment of schistosomiasis and liver fluke, although it is effective in other infections.[19]
Veterinary medicine
- Salmon poisoning disease
- Diplozoon paradoxum and other Trematoda infections of many fish species[20]
It may cause problems in dogs with MDR1 mutations.[21]
References
- ↑ "Farnam Pet Press Release. TRUSTED D-WORM™ offers product for tapeworm management". Farnam Companies, Inc. Retrieved 3 October 2016.
- 1 2 "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ Matthaiou DK, Panos G, Adamidi ES, Falagas ME (2008). Carabin, Hélène, ed. "Albendazole versus Praziquantel in the Treatment of Neurocysticercosis: A Meta-analysis of Comparative Trials". PLoS Negl Trop Dis. 2 (3): e194. doi:10.1371/journal.pntd.0000194. PMC 2265431
 . PMID 18335068.
. PMID 18335068. - 1 2 3 Drontal Data Sheet; Drontal Cat & Cat XL Film-coated Tablets, Bayer plc (PDF), Newbury, England: Bayer plc, Animal Health Division, p. 2, retrieved 23 September 2015
- 1 2 Bowman, Dwight D.; Hendrix, Charles M.; Lindsay, David S.; Barr, Steven C. (2002). Feline clinical parasitology (First ed.). Ames, Iowa: Iowa State University. p. 275. ISBN 0-8138-0333-0.
- ↑ Tchuenté LA, Shaw DJ, Polla L, Cioli D, Vercruysse J (December 2004). "Efficacy of praziquantel against Schistosoma haematobium infection in children". Am. J. Trop. Med. Hyg. 71 (6): 778–82. PMID 15642971.
- 1 2 The Carter Center. "Schistosomiasis Control Program". Retrieved 2008-07-17.
- ↑ Shen C, Kim J, Lee JK, et al. (June 2007). "Collection of Clonorchis sinensis adult worms from infected humans after praziquantel treatment". The Korean Journal of Parasitology. 45 (2): 149–52. doi:10.3347/kjp.2007.45.2.149. PMC 2526309
 . PMID 17570980.
. PMID 17570980. - ↑ Mas-Coma S, Bargues MD, Valero MA (October 2005). "Fascioliasis and other plant-borne trematode zoonoses". Int. J. Parasitol. 35 (11-12): 1255–78. doi:10.1016/j.ijpara.2005.07.010. PMID 16150452.
- ↑ Ridtitid W, Wongnawa M, Mahatthanatrakul W, Punyo J, Sunbhanich M (November 2002). "Rifampin markedly decreases plasma concentrations of praziquantel in healthy volunteers". Clin. Pharmacol. Ther. 72 (5): 505–13. doi:10.1067/mcp.2002.129319. PMID 12426514.
- ↑ Quinn DI, Day RO (June 1995). "Drug interactions of clinical importance. An updated guide". Drug Saf. 12 (6): 393–452. doi:10.2165/00002018-199512060-00005. PMID 8527014.
- ↑ Masimirembwa CM, Naik YS, Hasler JA (January 1994). "The effect of chloroquine on the pharmacokinetics and metabolism of praziquantel in rats and in humans". Biopharm Drug Dispos. 15 (1): 33–43. doi:10.1002/bdd.2510150103. PMID 8161714.
- ↑ Metwally A, Bennett JL, Botros S, Ebeid F (April 1995). "Effect of cimetidine, bicarbonate and glucose on the bioavailability of different formulations of praziquantel". Arzneimittelforschung. 45 (4): 516–8. PMID 7779153.
- ↑ Jung H, Medina R, Castro N, Corona T, Sotelo J (June 1997). "Pharmacokinetic study of praziquantel administered alone and in combination with cimetidine in a single-day therapeutic regimen". Antimicrob. Agents Chemother. 41 (6): 1256–9. PMC 163896
 . PMID 9174180.
. PMID 9174180. - ↑ http://bayer.naccvp.com/view_label.php
- ↑ Woelfle, M.; Seerden, J. P.; De Gooijer, J.; Pouwer, K.; Olliaro, P.; Todd, M. H. (2011). Geary, Timothy G, ed. "Resolution of Praziquantel". PLoS Neglected Tropical Diseases. 5 (9): e1260. doi:10.1371/journal.pntd.0001260. PMC 3176743
 . PMID 21949890.
. PMID 21949890. - ↑ "BILTRICIDE- praziquantel tablet, film coated (NDC Code(s): 50419-747-01)". DailyMed. July 2015. Retrieved 2015-09-08.
- ↑ "Antihelmintics - Medicines for Worms; threadword, roundworm". Patient. Retrieved 2015-06-15.
- ↑ Brunton, Laurence; Chabner, Bruce; Knollman, Bjorn (2011-01-10). Goodman and Gilman's The Pharmacological Basis of Therapeutics, Twelfth Edition (12 ed.). New York: McGraw-Hill Education / Medical. ISBN 9780071624428.
- ↑ "Treatment of fish parasites. 2. Effects of praziquantel, niclosamide, levamisole-HCl, and metrifonate on monogenea (Gyrodactylus aculeati, Diplozoon paradoxum)".
- ↑ http://www.collieassociation.co.uk/health/MDR1leaflet.pdf
External links
- "Praziquantel (Rx) Biltricide". Medscape. WebMD. Retrieved 2015-11-01.