Podophyllotoxin
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a684055 |
| Pregnancy category |
|
| ATC code | D06BB04 (WHO) |
| Pharmacokinetic data | |
| Biological half-life | 1.0 to 4.5 hours. |
| Identifiers | |
| |
| Synonyms | (5R,5aR,8aR,9R)-9-hydroxy-5-(3,4,5-trimethoxyphenyl)-5,8,8a,9-tetrahydrofuro[3',4':6,7]naphtho[2,3-d][1,3]dioxol-6(5aH)-one |
| CAS Number |
518-28-5 |
| PubChem (CID) | 10607 |
| DrugBank |
DB01179 |
| ChemSpider |
10162 |
| UNII |
L36H50F353 |
| KEGG |
D05529 |
| ChEBI |
CHEBI:50305 |
| ChEMBL |
CHEMBL61 |
| ECHA InfoCard | 100.007.502 |
| Chemical and physical data | |
| Formula | C22H22O8 |
| Molar mass | 414.405 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Podophyllotoxin (abbreviated as PPT), otherwise known as podofilox, is a non-alkaloid toxin lignan extracted from the roots and rhizomes of Podophyllum species.[1] Under the trade names Condylox, a gel, and Wartec, a solution or cream, it is used on the skin as a topical treatment of external genital warts, caused by some types of the human papillomavirus (HPV), and other warts. PPT and its synthetic derivatives display a wide selection in medical applications such as purgative, vesicant, antirheumatic, antiviral, and antitumor agents. These derivatives include etoposide and teniposide. Their anticancer activity has been heavily under study and used in various chemotherapies, including lung cancer, lymphomas, and genital tumors.
Historically, podophyllin, a resin extracted from Podophyllum and Sinopodophyllum species, was also used.
It is on the WHO Model List of Essential Medicines, the most important medications needed in a basic health system.[2]
Uses
Podophyllotoxin possesses a large number of medical applications and can be used as a cathartic, purgative, antiviral agent, vesicant, and antihelminthic. Additionally, podophyllotoxin and its derivatives are leads for anti-tumor agents such as etoposide and teniposide.[3][4]
Adverse effects
The most common side effects near the application site are skin reactions, including burning, redness, pain, itching, swelling.[5] Application can be immediately followed by burning or itching. Small sores, itching and peeling skin can also follow.[6]
Neither podophyllin resin nor podophyllotoxin lotions or gels are used during pregnancy because these medications can be harmful to the fetus.
Overdose and toxicity
While podophyllin, the herbal extract from which podophyllotoxin is derived, can cause enteritis and potentially fatal CNS depression, podophyllotoxin has been shown to be safe, with minimal toxicity even in large doses.[7]
Pharmacology
Mechanism of action
Podophyllotoxin and its derivatives display binding activity to the enzyme topoisomerase II during the late S and early G2 stage. For instance, etoposide binds and stabilizes the temporary DNA break caused by the enzyme, disrupts the reparation of the break through which the double-stranded DNA passes, and consequently stops DNA unwinding and replication.[8] Mutants resistant to either podophyllotoxin, or to its topoisomerase II inhibitory derivatives such as etoposide (VP-16), have been described in Chinese hamster cells.[9][10] The mutually exclusive cross-resistance patterns of these mutants provide a highly specific mean to distinguish the two kinds of podophyllotoxin derivatives.[10][11] Mutant Chinese hamster cells resistant to podophyllotoxin are affected in a protein P1 that was later identified as the mammalian HSP60 or chaperonin protein.[12][13][14]
Chemistry
Structural characteristic
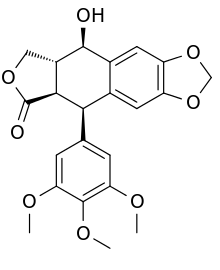
The structure of podophyllotoxin was first elucidated in the 1930s.[15] Podophyllotoxin bears four consecutive chiral centers, labelled C-1 through C-4 in the following image. The molecule also contains four almost planar fused rings. The podophyllotoxin molecule includes a number of oxygen containing functional groups: an alcohol, a lactone, three methoxy groups, and an acetal.[16]

Derivatives of podophyllotoxin are synthesized as properties of the rings and carbon 1 through 4 are diversified. For example, ring A is not essential to antimitotic activity. Aromatization of ring C leads to loss of activity, possibly from ring E no longer being placed on the axial position. In addition, the stereochemistry at C-2 and C-3 configures a trans-lactone, which has more activity than the cis counterpart. Chirality at C-1 is also important as it implies an axial position for ring E.[16]
Biosynthesis
The biosynthetic route of podophyllotoxin was not completely eludicidated for many years; however in September 2015, the identity of the six missing enzymes in podophyllotoxin biosynthesis were reported for the first time.[17] Several prior studies have suggested a common pathway starting from coniferyl alcohol being converted to (+)-pinoresinol in the presence of a one-electron oxidant [3] through dimerization of stereospecific radical intermediate. Pinoresinol is subsequently reduced in the presence of co-factor NADPH to first lariciresinol, and ultimately secoisolariciresinol. Lactonization on secoisolariciresinol gives rise to matairesinol. Secoisolariciresinol is assumed to be converted to yatein through appropriate quinomethane intermediates,[3] leading to podophyllotoxin.
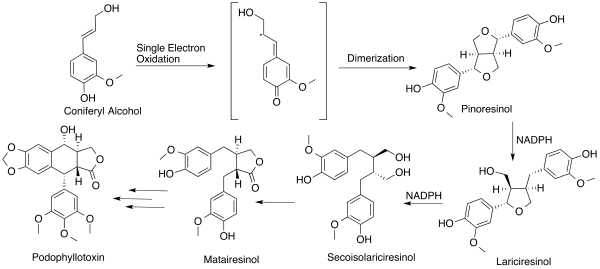
A sequence of enzymes involved has been reported to be dirigent protein (DIR), to convert coniferyl alcohol to (+)-pinocresol, which is converted by pinocresol-lariciresinol reductase (PLR) to (-)-secoisolariciresinol, which is converted by sericoisolariciresinol dehydrogenase (SDH) to (-)-matairesinol, which is converted by CYP719A23 to (-)-pluviatolide, which is likely converted by Phex13114 (OMT1) to (-)-yatein, which is converted by Phex30848 (2-ODD) to (-)-deoxypodophyllotoxin.[17] Though not proceeding through the last step of producing podophyllotoxin itself, a combination of six genes from the mayapple enabled production of the etoposide aglycone in tobacco plants.[17]
Natural abundance
It is present at concentrations of 0.3 to 1.0% by mass in the rhizome of American Mayapple (Podophyllum peltatum).[8][18] Another common source of podophyllotoxin is the rhizomes of Sinopodophyllum hexandrum Royle (Berberidaceae).
It is biosynthesized from two molecules of coniferyl alcohol by phenolic oxidative coupling and a series of oxidations, reductions and methylations.[8]
References
- ↑ Xu, H; Lv, M; Tian,X (2009). "A review on hemisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins: 2003-2007.". Current Medicinal Chemistry. 16 (3): 327–349. doi:10.2174/092986709787002682. PMID 19149581.
- ↑ "19th WHO Model List of Essential Medicines (April 2015)" (PDF). WHO. April 2015. Retrieved May 10, 2015.
- 1 2 3 Gordaliza M, García PA, del Corral JM, Castro MA, Gómez-Zurita MA (2004). "Podophyllotoxin: distribution, sources, applications and new cytotoxic derivatives". Toxicon. 44 (4): 441–59. doi:10.1016/j.toxicon.2004.05.008. PMID 15302526.
- ↑ Damayanthi Y, Lown JW (June 1998). "Podophyllotoxins: current status and recent developments". Curr. Med. Chem. 5 (3): 205–52. PMID 9562603.
- ↑ Longstaff E, von Krogh G (April 2001). "Condyloma eradication: self-therapy with 0.15-0.5% podophyllotoxin versus 20-25% podophyllin preparations--an integrated safety assessment". Regul. Toxicol. Pharmacol. 33 (2): 117–37. doi:10.1006/rtph.2000.1446. PMID 11350195.
- ↑ "PRODUCT INFORMATION WARTEC® SOLUTION" (PDF). GlaxoSmithKline Australia Pty Ltd. Retrieved 6 January 2013.
- ↑ von Krogh G, Longstaff E (December 2001). "Podophyllin office therapy against condyloma should be abandoned" (Registration required). Sex Transm Infect. 77 (6): 409–12. doi:10.1136/sti.77.6.409. PMC 1744412
 . PMID 11714936.
. PMID 11714936. - 1 2 3 Canel, C; Moraes, RM; Dayan, FE; Ferreira, D (2000). "Molecules of Interest: Podophyllotoxin". Phytochemistry. 54 (2): 115–120. doi:10.1016/s0031-9422(00)00094-7.
- ↑ Gupta, R.S.; Ho, T.K.W.; Moffat, M.R.K.; Gupta, R. (1982). "Podophyllotoxin-resistant mutants of Chinese hamster ovary cells. Alteration in a microtubule-associated protein". J. Biol. Chem. 257: 1071–1078.
- 1 2 Gupta, R.S. (1983). "Genetic, biochemical and cross-resistance studies with mutants of Chinese hamster ovary cells resistant to the anticancer drugs VM-26 and VP16-213". Cancer Res. 43: 1568–1574.
- ↑ Gupta, R.S. (1983). "Podophyllotoxin resistant mutants of Chinese hamster ovary cells: Cross resistance studies with various microtubule inhibitors and podophyllotoxin analogs". Cancer Res. 43: 505–512.
- ↑ Picketts, D.J.; Mayanil, C.S.K.; Gupta, R.S. (1989). "Molecular cloning of a Chinese hamster mitochondrial protein related to the "chaperonin" family of bacterial and plant proteins". J. Biol. Chem. 264: 12001–12008.
- ↑ Jindal, S.; Dudani, A.K.; Singh, B.; Harley, C.B.; Gupta, R.S (1989). "Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65 kDa mycobacterial antigen". Mol. Cell. Biol. 9: 2279–2283. doi:10.1128/mcb.9.5.2279.
- ↑ Anthony J. Trevor, Bertram G. Katzung, Marieke Kruidering-Hall, Susan B. Masters. Chapter 54: Cancer Chemotherapy. Katzung & Trevor's Pharmacology: Examination & Board Review, 10th edition.
- ↑ Borsche, W.; Niemann J. (1932). "Über Podophyllin". Justus Liebigs Ann. Chem. 494: 126–142. doi:10.1002/jlac.19324940113.
- 1 2 You, Y (2005). "Podophyllotoxin derivatives: current synthetic approaches for new anticancer agents.". Current Pharmaceutical Design. 11 (13): 1695–1717. doi:10.2174/1381612053764724. PMID 15892669.
- 1 2 3 Lau, Warren; Sattely, Elizabeth S. "Six enzymes from the mayapple that complete the biosynthetic pathway to the etoposide aglycone". Science. 349 (6253): 1224–1228. doi:10.1126/science.aac7202.
- ↑ J. L. Hartwell; A. W. Schrecker (1951). "Components of Podophyllin. V. The Constitution of Podophyllotoxin". Journal of the American Chemical Society. 73 (6): 2909–2916. doi:10.1021/ja01150a143.
Further reading
- Kelly M; Hartwell J. L. (1954). "The biological effects and the chemical composition of podophyllin: a review". Journal of the National Cancer Institute. 14 (4): 967–1010. PMID 13233838.
- J. L. Hartwell; A. W. Schrecker (1951). "Components of Podophyllin. V. The Constitution of Podophyllotoxin". Journal of the American Chemical Society. 73 (6): 2909–2916. doi:10.1021/ja01150a143.