Edoxudine
 | |
| Clinical data | |
|---|---|
| ATC code | D06BB09 (WHO) |
| Identifiers | |
| |
| CAS Number |
15176-29-1 |
| PubChem (CID) | 66377 |
| ChemSpider |
59752 |
| UNII |
15ZQM81Y3R |
| ChEMBL |
CHEMBL318153 |
| ECHA InfoCard | 100.035.645 |
| Chemical and physical data | |
| Formula | C11H16N2O5 |
| Molar mass | 256.25514 |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
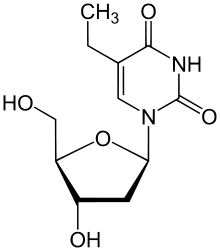
Edoxudine (or edoxudin) is an antiviral drug. It is an analog of thymidine, a nucleoside.
It has shown effectiveness against herpes simplex virus.[1]
Synthesis
Mercuration of the 2'-deoxyuridine 1 leads to the organometallic derivative 2; reaction of that with ethylene in the presence dilithiopalladium tetrachloride gives the alkylation product 3; this is reduced catalytically in situ. There is thus obtained the antiviral agent edoxudine 4.
References
- ↑ "Topical antiviral agents for herpes simplex virus infections". Drugs Today. 34 (12): 1013–25. December 1998. doi:10.1358/dot.1998.34.12.487486. PMID 14743269.
- ↑ K. K. Gauri, GB 1170565; eidem, U.S. Patent 3,553,192 (1968, 1971 both to Robugen).
- ↑ Bergstrom, Donald E.; Ruth, Jerry L. (1976). "Synthesis of C-5 substituted pyrimidine nucleosides via organopalladium intermediates". Journal of the American Chemical Society. 98 (6): 1587–9. doi:10.1021/ja00422a056. PMID 1249369.
- ↑ Bergstrom, Donald E.; Ogawa, Mark K. (1978). "C-5 substituted pyrimidine nucleosides. 2. Synthesis via olefin coupling to organopalladium intermediates derived from uridine and 2'-deoxyuridine". Journal of the American Chemical Society. 100 (26): 8106–8112. doi:10.1021/ja00494a014. ISSN 0002-7863.
This article is issued from Wikipedia - version of the 4/10/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
